在体外受精-胚胎移植(in vitro fertilization-embryo transfer,IVF-ET)周期中,受精异常的情况时常发生,其中形成多原核(pronuclei,PN)的合子一般不用于胚胎移植,造成了卵母细胞和胚胎的浪费[1]。如果患者多PN率较高,可能会因为无正常胚胎可移植而造成临床妊娠失败。并且,移植发生多PN胚胎会使患者流产风险增加,约占自然流产的细胞遗传学异常病例的15%~18%,也说明发生多PN胚胎质量较差[2]。多囊卵巢综合征(polycystic ovarian syndrome,PCOS)患者抗苗勒管激素(anti-Mullerian hormone,AMH)水平较高,多PN受精卵数量在AMH水平较高时增加[3]。AMH由卵巢颗粒细胞合成,始于初级卵泡,在腔前卵泡和小卵泡中最高,在大卵泡中减少,不仅能够作为卵巢储备的可靠指标,还参与PCOS的发病机制[4]。PCOS患者小窦卵泡发育成熟受阻,导致排卵障碍,是无排卵性不孕的主要病因,IVF-ET可作为有效的治疗手段。但往往因为患者生殖-代谢紊乱,卵母细胞质量较差,而增加多PN形成的可能性[5]。目前关于多PN对PCOS患者IVF妊娠结局和围产期结局的影响报道较少,本研究进行回顾性分析,旨在探讨PCOS患者中多PN的发生对冻融胚胎移植(frozen-thawed embryo transfer,FET)妊娠结局和围产期结局的影响。期望能改善临床PCOS患者的胚胎移植策略,减少PCOS患者多PN发生率,提高其卵母细胞利用率,增加患者临床妊娠的机会。
资料与方法
一、研究资料收集
回顾性分析2016年1月至2021年12月于南京医科大学附属妇产医院进行IVF患者的治疗资料。分析PCOS人群的多PN发生率,纳入标准:(1)受精方式为IVF,治疗方案为长方案或者拮抗剂方案;(2)不孕因素为输卵管因素(输卵管炎、输卵管阻塞及输卵管切除)或者诊断为PCOS(根据鹿特丹标准[6]诊断);(3)基础病史资料完整。排除标准:(1)子宫异常,包括子宫畸形、子宫内膜息肉等;(2)卵巢异常,包括单侧卵巢缺如、卵巢囊肿等;(3)接受植入前遗传学筛查的夫妇;(4)丈夫或妻子染色体异常;(5)其他引起排卵障碍以及高雄激素血症的疾病;(6)内分泌疾病;(7)免疫性疾病。共纳入患者2 665例,根据不孕因素分为PCOS组(PCOS,N=1 295)和对照组(输卵管因素,N=1 370)。
对上述1 295例PCOS患者资料进行更为深入的分析,探讨多PN发生的危险因素及对妊娠结局的影响时,新增纳入标准为选取其中全胚冷冻并进行FET的患者,并排除妊娠结局失访或未到妊娠时间的病例。最终纳入患者999例,根据患者IVF周期的受精情况分为三个亚组:正常受精2PN组(多PN率=0%,N=547),低比例多PN组(多PN率≤20%,N=414)和高比例多PN组(多PN率>20%,N=38)。
二、研究方法
1. 超促排卵和取卵:所有患者于月经第2~3 d检查内分泌水平,并测量基础窦卵泡计数,本科室根据国际标准化组织(International Standards Organization,ISO)标准化流程制定促排卵方案。当至少2枚卵泡直径≥18.0 mm时,结合血E2、LH和孕酮水平,注射人绒毛膜促性腺激素(human chorionic gonadotropin,HCG)5 000.0~10 000.0 U诱发排卵,HCG日36 h后在阴道超声监测下取卵。
2.精液处理和体外受精:取卵当日男方采用自慰方式留取精液标本,然后采用密度梯度离心法处理精液。IVF后置于G1 plus培养液(瑞典Vitrolife公司)中进行培养,第1 d观察并记录受精情况。正常受精的胚胎继续培养至第3 d或第5~6 d行全胚冷冻。卵裂期胚胎质量分为4个等级:Ⅰ级,卵裂球形状规则,大小均匀,DNA碎片不明显;Ⅱ级,卵裂球大小略不均匀,形态稍微不规则,DNA碎片率<20%;Ⅲ级,卵裂球大小明显不均,DNA碎片率20%~50%;Ⅳ级,DNA碎片率>50%。囊胚评分标准参考 Gardner 评分系统[7],囊胚评分≥3 BB及以上均视为优质囊胚。
3.内膜准备方案和胚胎移植:月经周期第2~3 d行阴道B超和性激素检查,结合检查结果和患者意愿选择子宫内膜准备方案。(1)自然周期方案:适用于有规律月经周期及正常排卵者。月经周期第10~12 d监测卵泡生长和子宫内膜。当卵泡直径>18.0 mm,子宫内膜厚度≥7.0 mm时,肌内注射10 000.0 U HCG。(2)人工周期方案:适用于月经周期不规律、排卵障碍、自然周期子宫内膜生长不良患者。月经周期第2 d口服雌激素,当子宫内膜厚度达到7.0 mm,血清雌二醇水平达到200.0 pg/mL时,给予孕激素转化内膜。采用玻璃化方案进行胚胎冷冻复苏,根据患者体质指数(BMI)、胚胎质量、是否瘢痕子宫以及个人意愿等情况综合考评后选择移植1~2枚胚胎。
4. 随访:移植后14 d检测血清β-hCG,若β-hCG>50.0 U/L,持续黄体期支持至妊娠8~10周,妊娠6周后经B超检查确认孕囊或原始心管搏动,每2周B超检查一次,至妊娠8~10周,中心专人电话随访妊娠中晚期及分娩情况。
三、观察指标和诊断标准
1. 观察指标:根据本中心病例系统收集所需相关信息,统计指标包括:(1)一般资料,包括夫妻双方年龄和BMI、妊娠次数、生产次数等;(2)胚胎发育情况,包括获卵数、卵裂数、囊胚形成数等;(3)胚胎移植、妊娠结局和围产期结局,包括胚胎种植率、生化妊娠率、临床妊娠率、流产率等。
2. 诊断标准:妊娠后未满12周的妊娠丢失为早期流产,发生在12周后但未达到28周的妊娠丢失为晚期流产;妊娠满28周但未满37周分娩为早产,达到37周而未满42周分娩为足月产;新生儿出生体质量<2 500.0 g为低出生质量儿,>4 000.0 g为巨大儿。
四、统计学方法
数据分析采用SPSS 26.0统计学软件。正态分布的定量数据,以均数±标准差![]() 表示,独立样本t检验行组间比较;不符合正态分布的定量资料,进行非参数检验。定性资料用构成比或率(%)表示,组间比较行χ2检验。采用多因素二元Logistic回归分析影响多PN发生以及妊娠结局的因素,采用多因素多元Logistic回归分析影响多PN率的因素。P<0.05为差异有统计学意义。
表示,独立样本t检验行组间比较;不符合正态分布的定量资料,进行非参数检验。定性资料用构成比或率(%)表示,组间比较行χ2检验。采用多因素二元Logistic回归分析影响多PN发生以及妊娠结局的因素,采用多因素多元Logistic回归分析影响多PN率的因素。P<0.05为差异有统计学意义。
结 果
一、PCOS组和对照组患者的临床资料比较
PCOS组和对照组的男方BMI、处理后前向运动精子、授精时间长度差异无统计学意义(P>0.05),其余指标差异均有统计学意义(P<0.05)。见表1。
表1 PCOS组和对照组患者的临床资料比较![]()
Table 1 Comparison of clinical data between patients in PCOS group and control ![]()
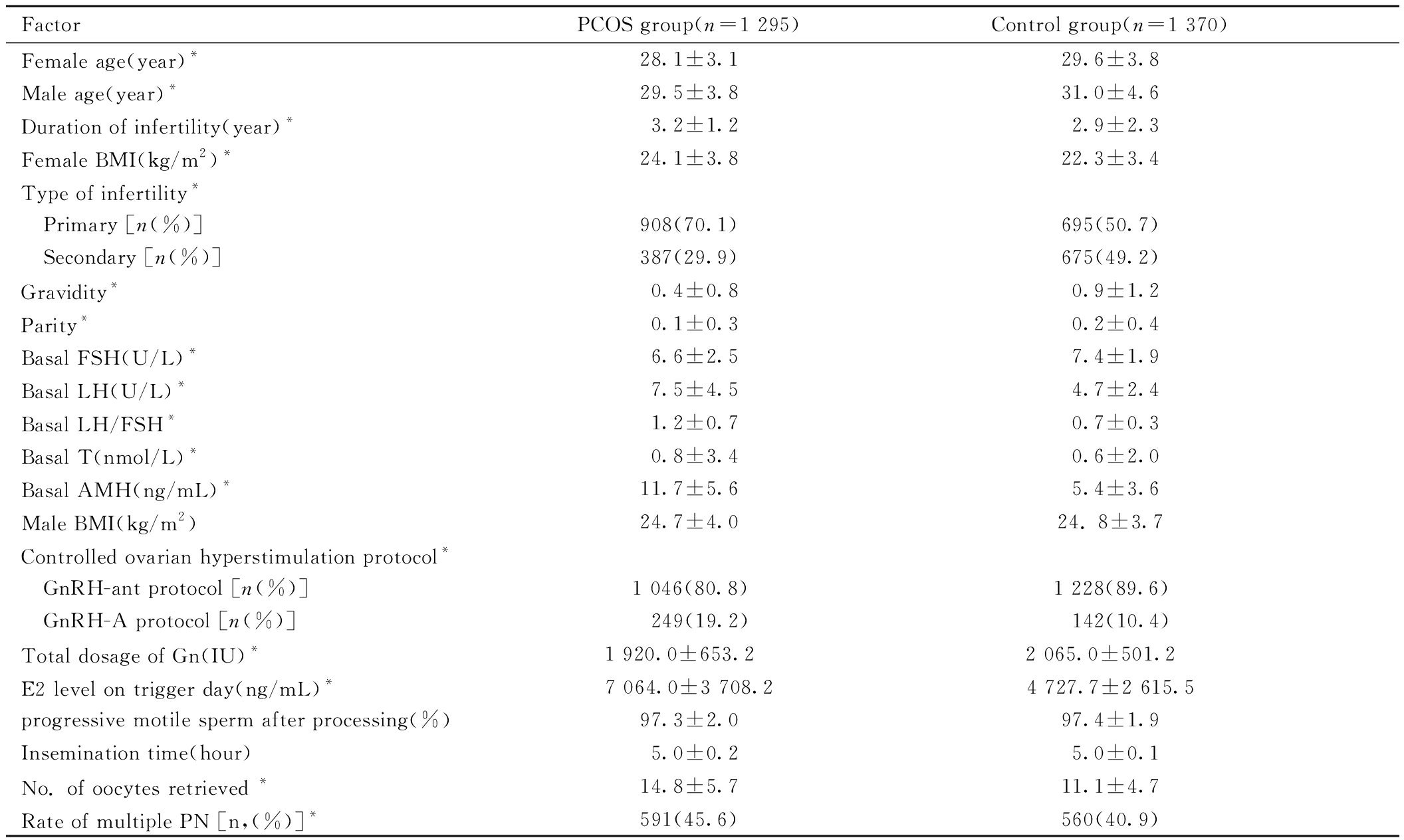
FactorPCOS group(n=1 295)Control group(n=1 370)Female age(year)*28.1±3.129.6±3.8Male age(year)*29.5±3.831.0±4.6Duration of infertility(year)*3.2±1.22.9±2.3Female BMI(kg/m2)*24.1±3.822.3±3.4Type of infertility* Primary [n(%)]908(70.1)695(50.7) Secondary [n(%)]387(29.9)675(49.2)Gravidity*0.4±0.80.9±1.2Parity*0.1±0.30.2±0.4Basal FSH(U/L)*6.6±2.57.4±1.9Basal LH(U/L)*7.5±4.54.7±2.4Basal LH/FSH*1.2±0.70.7±0.3Basal T(nmol/L)*0.8±3.40.6±2.0Basal AMH(ng/mL)*11.7±5.65.4±3.6Male BMI(kg/m2)24.7±4.024. 8±3.7Controlled ovarian hyperstimulation protocol* GnRH-ant protocol [n(%)]1 046(80.8)1 228(89.6) GnRH-A protocol [n(%)] 249(19.2) 142(10.4)Total dosage of Gn(IU)*1 920.0±653.2 2 065.0±501.2 E2 level on trigger day(ng/mL)*7 064.0±3 708.24 727.7±2 615.5progressive motile sperm after processing(%)97.3±2.097.4±1.9Insemination time(hour)5.0±0.25.0±0.1No. of oocytes retrieved *14.8±5.711.1±4.7Rate of multiple PN [n,(%)]*591(45.6)560(40.9)
Note:compared with two groups, *P<0.05. PCOS represents polycystic ovarian syndrome; BMI represents body mass index; FSH represents Follicle stimulating hormone; LH represents Luteinizing hormone; T represents Testosterone; AMH represents Anti-Mullerian Hormone; Gn represents gonadotropin; E2 represents Estradiol; PN represents pronuclei.
运用多因素二元Logistic回归排除女方年龄、不孕类型、不孕年限、妊娠次数、生产次数、女性BMI混杂因素后,PCOS组的多PN发生率明显高于对照组(aOR=1.21,95%CI:1.02~1.43),PCOS是多PN发生的危险因素。见表2。
表2 多PN发生的多因素二元Logistic回归
Table 2 Multivariate binary logistic regression analysis of multiple PN occurrence.

FactornRate of multiple PN [n(%)]aOR(95%CI)PCOS group(vs control group)1 295591(45.6)1.21(1.02-1.43)GnRH-A protocol(vs GnRH-ant protocol) 391190(48.6)1.27(1.02-1.59)
Note:PN represents pronulei; aOR represents adjusted Odds Ratio; 95%CI represents 95% confidence interval; PCOS represents polycystic ovarian syndrome.
二、PCOS组内各亚组患者的临床资料比较
PCOS内各亚组之间孕次、不孕类型、基础AMH、hCG日E2、获卵数差异有统计学意义(P<0.05),其他指标无统计学意义(P>0.05)。见表3。
表3 PCOS组内各亚组患者的临床资料比较![]()
Table 3 Clinical data comparison of patients in different subgroups within the PCOS ![]()
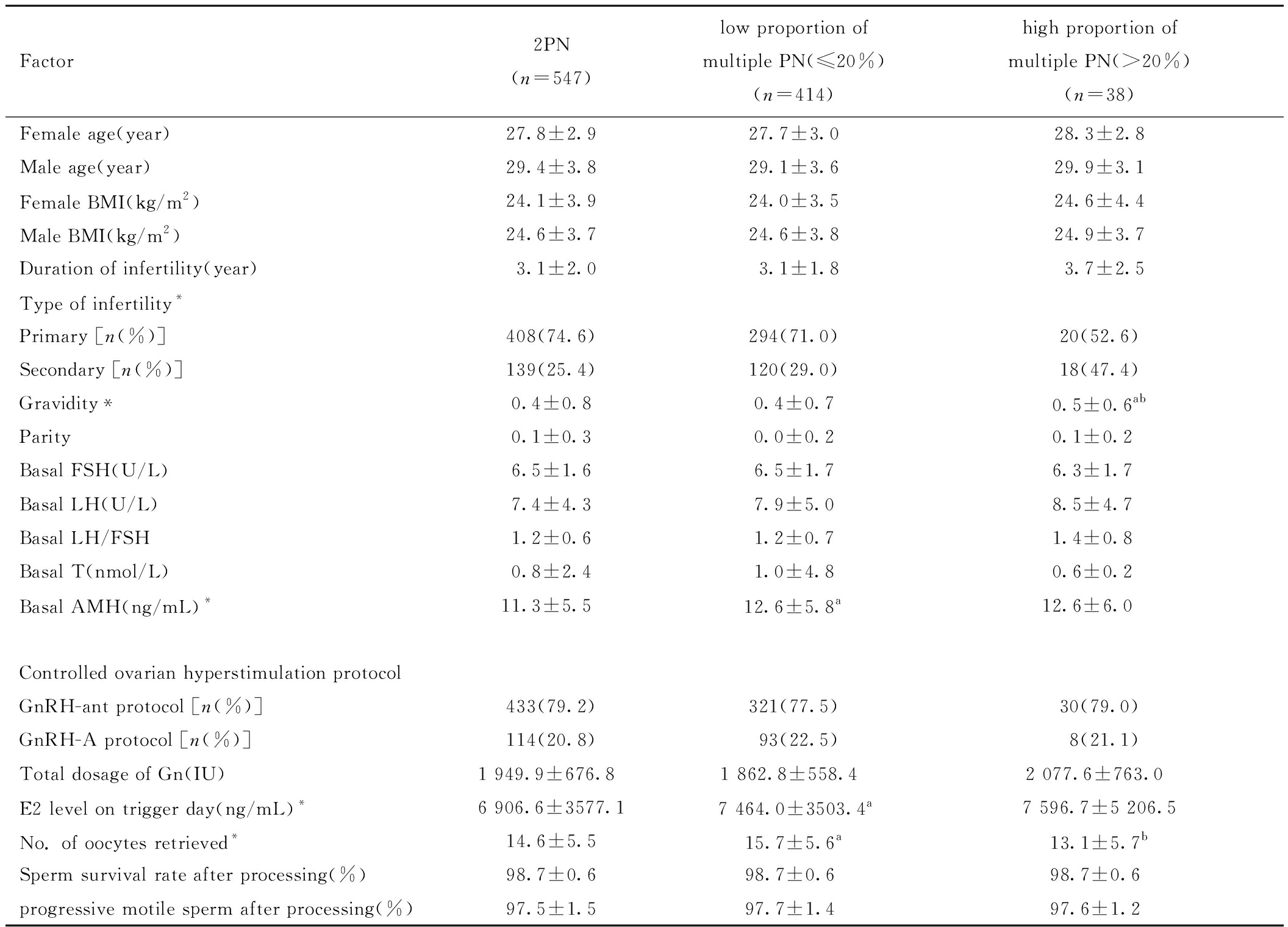
Factor2PN(n=547)low proportion of multiple PN(≤20%)(n=414)high proportion of multiple PN(>20%)(n=38)Female age(year)27.8±2.927.7±3.028.3±2.8Male age(year)29.4±3.829.1±3.629.9±3.1Female BMI(kg/m2)24.1±3.924.0±3.524.6±4.4Male BMI(kg/m2)24.6±3.724.6±3.824.9±3.7Duration of infertility(year)3.1±2.03.1±1.83.7±2.5Type of infertility*Primary [n(%)]408(74.6)294(71.0)20(52.6)Secondary [n(%)]139(25.4)120(29.0)18(47.4)Gravidity*0.4±0.80.4±0.70.5±0.6abParity0.1±0.30.0±0.20.1±0.2Basal FSH(U/L)6.5±1.66.5±1.76.3±1.7Basal LH(U/L)7.4±4.37.9±5.08.5±4.7Basal LH/FSH1.2±0.61.2±0.71.4±0.8Basal T(nmol/L)0.8±2.41.0±4.80.6±0.2Basal AMH(ng/mL)*11.3±5.512.6±5.8a12.6±6.0Controlled ovarian hyperstimulation protocolGnRH-ant protocol [n(%)]433(79.2)321(77.5)30(79.0)GnRH-A protocol [n(%)]114(20.8)93(22.5)8(21.1)Total dosage of Gn(IU)1 949.9±676.8 1 862.8±558.4 2 077.6±763.0 E2 level on trigger day(ng/mL)*6 906.6±3577.17 464.0±3503.4a7 596.7±5 206.5No. of oocytes retrieved*14.6±5.515.7±5.6a13.1±5.7bSperm survival rate after processing(%)98.7±0.698.7±0.698.7±0.6progressive motile sperm after processing(%)97.5±1.597.7±1.497.6±1.2
Note:*P<0.05 compared between the three groups; a P<0.05 compared with 2PN group; b P<0.05 compared with low proportion of multiple PN(≤20%) group. PN represents pronuclei; BMI represents body mass index; FSH represents Follicle stimulating hormone; LH represents Luteinizing hormone; T represents Testosterone; AMH represents Anti-Mullerian Hormone; Gn represents gonadotropin; E2 represents Estradiol.
进行多因素多元Logistic回归后排除女方BMI、Gn启动总剂量、处理后精子存活率、妊娠次数、促排卵方案混杂因素后,结果显示相对于2PN组,低比例多PN组的基础AMH水平高、获卵总数较高(aOR= 1.04,95%CI:1.01~0.06;aOR= 1.03,95%CI:1.01~1.06),高比例多PN组的基础LH/FSH值较高(aOR= 1.78,95%CI:1.10~2.87)。见表4。
表4 PCOS组内各亚组间多PN率的多因素多元Logistic回归
Table 4 Multivariate logistic regression analysis of multiple PN rates among subgroups within the PCOS group
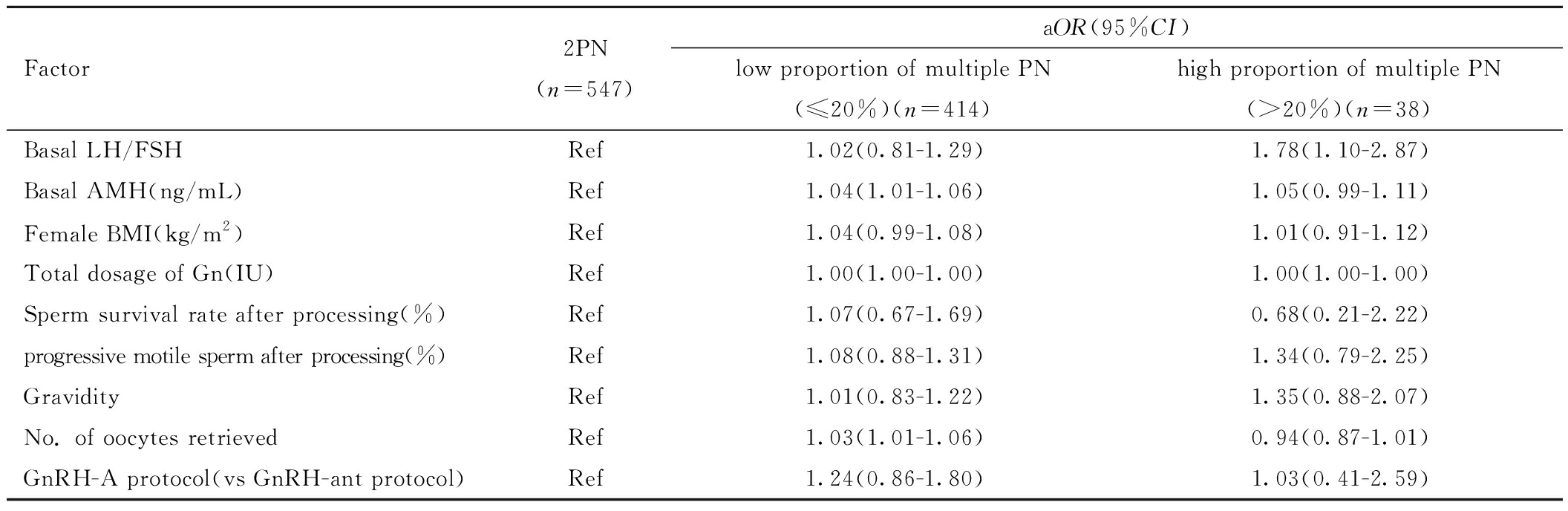
Factor2PN(n=547)aOR(95%CI)low proportion of multiple PN(≤20%)(n=414)high proportion of multiple PN(>20%)(n=38)Basal LH/FSHRef1.02(0.81-1.29)1.78(1.10-2.87)Basal AMH(ng/mL)Ref1.04(1.01-1.06)1.05(0.99-1.11)Female BMI(kg/m2)Ref1.04(0.99-1.08)1.01(0.91-1.12)Total dosage of Gn(IU)Ref1.00(1.00-1.00)1.00(1.00-1.00)Sperm survival rate after processing(%)Ref1.07(0.67-1.69)0.68(0.21-2.22)progressive motile sperm after processing(%)Ref1.08(0.88-1.31)1.34(0.79-2.25)GravidityRef1.01(0.83-1.22)1.35(0.88-2.07)No. of oocytes retrievedRef1.03(1.01-1.06)0.94(0.87-1.01)GnRH-A protocol(vs GnRH-ant protocol)Ref1.24(0.86-1.80)1.03(0.41-2.59)
Note:PN represents pronuclei; aOR represents adjusted Odds Ratio; CI represents confidence interval; BMI represents body mass index; FSH represents Follicle stimulating hormone; LH represents Luteinizing hormone; AMH represents Anti-Mullerian Hormone; Gn represents gonadotropin.
三、PCOS组内各亚组患者的实验室结局比较
各亚组间优质囊胚率没有差异(P均>0.05),其余指标差异均有统计学意义(P<0.05)。和2PN组相比,虽然低比例多PN组获卵数多,但其他各类指标呈下降趋势,且高比例多PN组低于低比例多PN组。见表5。
表5 PCOS组内各亚组患者的实验室结局比较![]()
Table 5 Comparison of laboratory outcomes among subgroups of patients in the PCOS ![]()
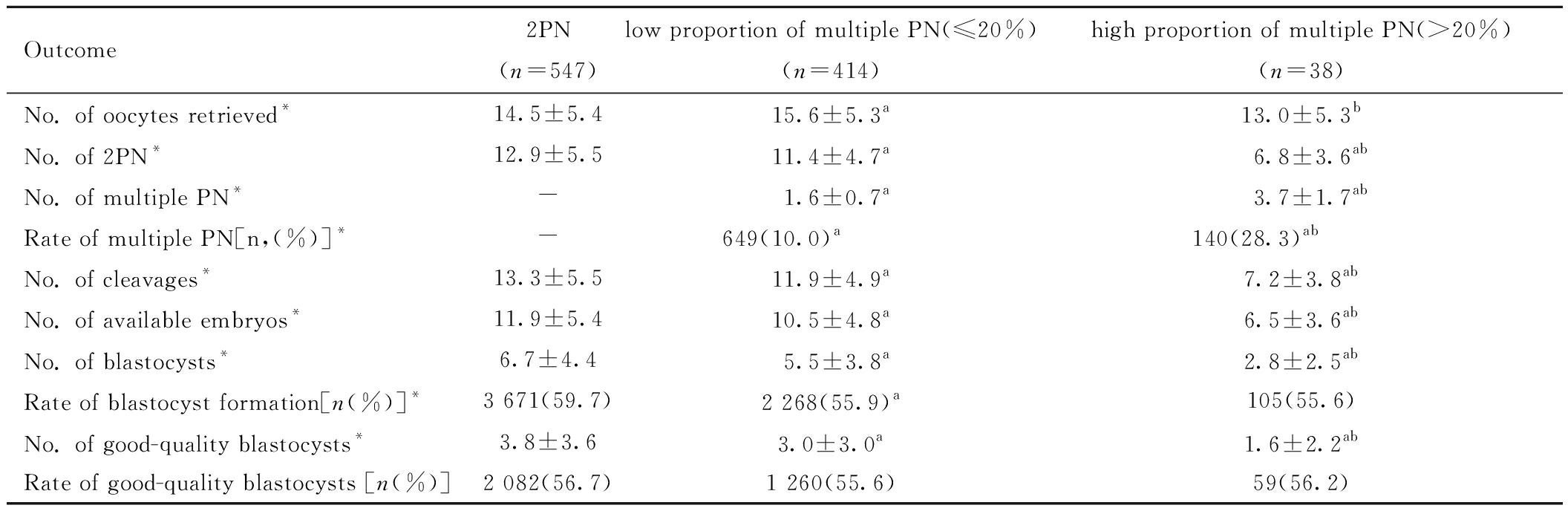
Outcome2PN(n=547)low proportion of multiple PN(≤20%)(n=414)high proportion of multiple PN(>20%)(n=38)No. of oocytes retrieved*14.5±5.415.6±5.3a13.0±5.3bNo. of 2PN*12.9±5.511.4±4.7a6.8±3.6abNo. of multiple PN*-1.6±0.7a3.7±1.7abRate of multiple PN[n,(%)]*-649(10.0)a140(28.3)abNo. of cleavages*13.3±5.511.9±4.9a7.2±3.8abNo. of available embryos*11.9±5.410.5±4.8a6.5±3.6abNo. of blastocysts*6.7±4.45.5±3.8a2.8±2.5abRate of blastocyst formation[n(%)]*3 671(59.7)2 268(55.9)a105(55.6)No. of good-quality blastocysts*3.8±3.63.0±3.0a1.6±2.2abRate of good-quality blastocysts [n(%)]2 082(56.7) 1 260(55.6)59(56.2)
Note:* P<0.05 compared between the three groups; a P<0.05 compared with 2PN group; b P<0.05 compared with low proportion of multiple PN(≤20%) group. PN represents pronuclei.
四、PCOS组内各亚组患者的胚胎移植情况和妊娠结局比较
各亚组间移植胚胎数、移植优质胚胎数、内膜准备方案无差异(P均>0.05)。移植胚胎类型差异有统计学意义(P<0.05)。各亚组间生化妊娠率、临床妊娠率、活产率间差异有统计学意义(P<0.05)。见表6。
表6 PCOS组内各亚组患者的胚胎移植情况和妊娠结局比较
Table 6 Comparison of embryo transfer data and pregnancy outcomes among subgroups of patients in the PCOS group
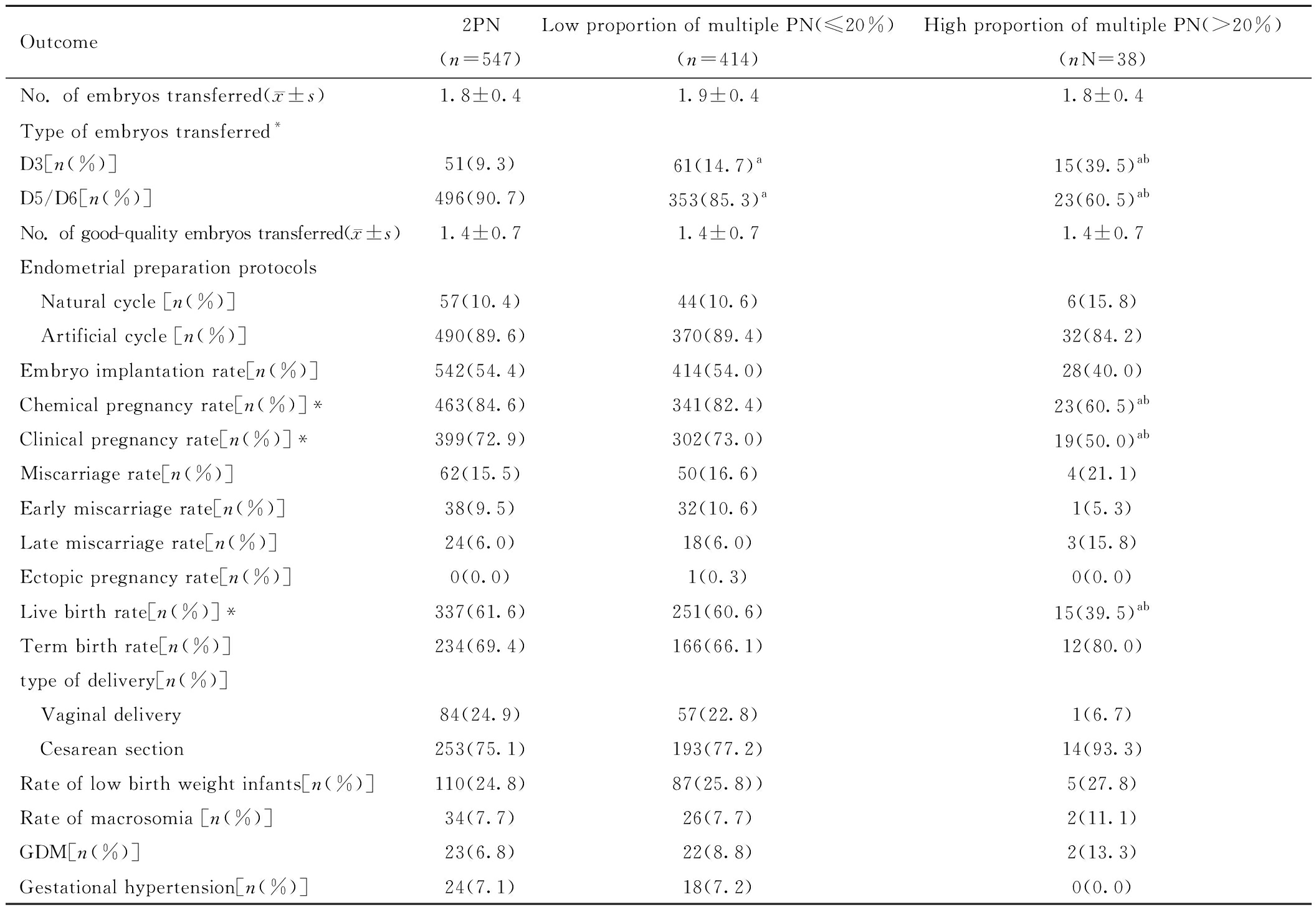
Outcome2PN(n=547)Low proportion of multiple PN(≤20%)(n=414)High proportion of multiple PN(>20%)(nN=38)No. of embryos transferred(x±s)1.8±0.41.9±0.41.8±0.4Type of embryos transferred*D3[n(%)]51(9.3)61(14.7)a15(39.5)abD5/D6[n(%)]496(90.7)353(85.3)a23(60.5)abNo. of good-quality embryos transferred(x±s)1.4±0.71.4±0.71.4±0.7Endometrial preparation protocols Natural cycle [n(%)]57(10.4)44(10.6)6(15.8) Artificial cycle [n(%)]490(89.6)370(89.4)32(84.2)Embryo implantation rate[n(%)]542(54.4)414(54.0)28(40.0)Chemical pregnancy rate[n(%)]*463(84.6)341(82.4)23(60.5)abClinical pregnancy rate[n(%)]*399(72.9)302(73.0)19(50.0)abMiscarriage rate[n(%)]62(15.5)50(16.6)4(21.1)Early miscarriage rate[n(%)]38(9.5)32(10.6)1(5.3)Late miscarriage rate[n(%)]24(6.0)18(6.0)3(15.8)Ectopic pregnancy rate[n(%)]0(0.0)1(0.3)0(0.0)Live birth rate[n(%)]*337(61.6)251(60.6)15(39.5)abTerm birth rate[n(%)]234(69.4)166(66.1)12(80.0)type of delivery[n(%)] Vaginal delivery84(24.9)57(22.8)1(6.7) Cesarean section253(75.1)193(77.2)14(93.3)Rate of low birth weight infants[n(%)]110(24.8)87(25.8))5(27.8)Rate of macrosomia [n(%)]34(7.7)26(7.7)2(11.1)GDM[n(%)]23(6.8)22(8.8)2(13.3)Gestational hypertension[n(%)]24(7.1)18(7.2)0(0.0)
Note:*P<0.05 compared between the three groups; a P<0.05 compared with 2PN group; b P<0.05 compared with low proportion of multiple PN(≤20%) group. PN represents pronuclei. GDM represents Gestational Diabetes Mellitus.
多因素二元Logistic回归分析影响PCOS患者生化妊娠、临床妊娠和活产的因素(定义获得生化妊娠、临床妊娠、活产均为1)发现,PCOS患者中2PN组(aOR=3.86,95%CI:1.74~8.54;aOR=2.35,95%CI:1.13~4.85)及低比例多PN组(aOR=2.98,95%CI:1.34~6.60;aOR=2.20,95%CI:1.06~4.58)的生化妊娠率及活产率显著高于多比例多PN组(Ref)。此外,BMI升高影响获得生化妊娠率及活产率(aOR=0.95,95%CI:0.90~0.99;aOR= 0.96,95%CI:0.92~0.99);而优质胚胎数增加可提高获得生化妊娠率、临床妊娠率及活产率(aOR=1.82,95%CI:1.42~2.33;aOR=2.21,95%CI:1.70~2.88;aOR=1.84,95%CI:1.51~2.23)。见表7。
表7 各亚组间妊娠结局的多因素二元Logistic回归分析,aOR(95%CI)
Table 7 Multivariate binary logistic regression analysis of pregnancy outcomes among different subgroups
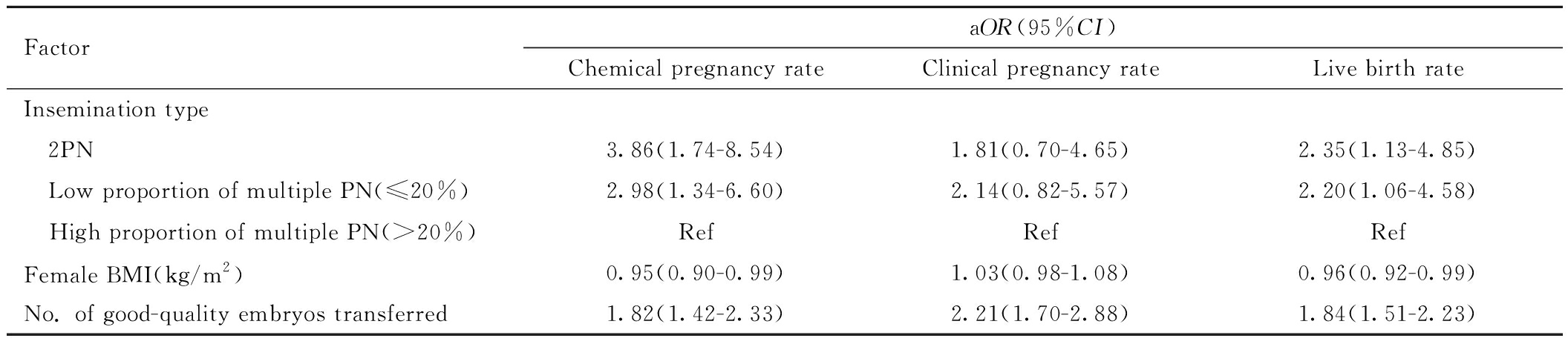
FactoraOR(95%CI)Chemical pregnancy rateClinical pregnancy rateLive birth rateInsemination type 2PN3.86(1.74-8.54)1.81(0.70-4.65)2.35(1.13-4.85) Low proportion of multiple PN(≤20%)2.98(1.34-6.60)2.14(0.82-5.57)2.20(1.06-4.58) High proportion of multiple PN(>20%)RefRefRefFemale BMI(kg/m2)0.95(0.90-0.99)1.03(0.98-1.08)0.96(0.92-0.99)No. of good-quality embryos transferred1.82(1.42-2.33)2.21(1.70-2.88)1.84(1.51-2.23)
Note:aOR represents adjusted Odds Ratio; CI represents confidence interval; PN represents pronuclei. BMI represents body mass index.
讨 论
PCOS是一种影响整个生命周期的生殖、代谢和心理的异质性内分泌疾病,全球约5%~18%的女性受到该病威胁[8],PCOS因素不孕患者占不孕症患者的21%~51%[9]。PCOS患者会出现排卵障碍、子宫内膜容受性下降及卵母细胞质量异常,同时伴随一系列内分泌异常,可能会导致患者受孕困难,IVF-ET是PCOS患者常用的助孕方法。有文献报道,PCOS患者的正常受精率低于对照组,也有研究报道高比例的多PN率可能会使总体胚胎质量下降[10-11],因此,本研究首先探讨PCOS是否更容易发生多PN。
本研究选取仅有输卵管因素的不孕症患者作为对照组,发现PCOS不孕患者多PN率显著高于对照组,且在校正混杂因素后,显示PCOS仍然是发生多PN的高危因素(aOR=1.21,95%CI:1.02~1.43)。一般认为,多PN发生可能由于多精入卵、卵母细胞第二极体排出失败或多倍体精卵融合,其发生与卵母细胞的成熟息息相关[12]。卵母细胞在受精过程中主要通过向卵周间隙释放皮质颗粒防止多精受精,而不成熟卵母细胞不能正常释放皮质颗粒,无法阻止多精子进入[13-14]。一项基础研究证明,PCOS与卵泡发育不良及卵母细胞-颗粒细胞通讯受损密切相关,导致PCOS患者卵母细胞质量低于正常妇女[15]。且PCOS患者在促排卵过程中,在Gn刺激下会募集过多的卵泡,但部分卵泡并不能够发育到成熟卵泡大小,因此扳机时未成熟卵母细胞比例高于正常女性,可能是PCOS患者多PN发生率高的原因。
目前关于多PN受精卵能否反映卵母细胞质量以及剩余正常受精胚胎的发育潜能和临床妊娠结局的研究数量不多,存在争议,且并没有关注PCOS人群[16-20]。因此,本研究分析了PCOS中正常受精组,低比例多PN组和高比例多PN组多PN发生的可能高危因素及对同周期剩余胚胎的发育及移植后妊娠结局的影响。本文中PCOS中低比例多PN患者呈现AMH水平高、扳机日E2水平高、获卵多的特点。尽管低比例多PN患者获卵数高,但剩余胚胎在培养后,可移植胚胎数、囊胚形成率和优质囊胚率均低于正常受精组,且移植后生化妊娠率及活产率显著降低、流产率较高。可能提示低比例多PN的PCOS患者卵巢储备条件和卵巢反应条件较好,经过促排卵治疗后更容易募集较多数量的卵泡,但扳机时存在未成熟卵泡,导致未成熟卵母细胞较多,因此更易出现多PN,即使正常受精形成胚胎,也会影响胚胎质量,从而对妊娠结局造成不利影响。
与低比例多PN患者相反,高比例多PN患者获卵数较正常受精组少,仅表现为LH/FSH比例高的特点。同时,相较于正常受精组,高比例多PN组可移植胚胎数、囊胚形成数、囊胚形成率及优质囊胚数明显降低。高比例多PN组的生化妊娠率和活产率也显著低于正常受精组和低比例多PN组,流产率较正常受精组和低比例多PN组高。有多项研究显示,PCOS患者中,基础高LH水平会促使卵泡膜细胞分泌睾酮,导致卵子质量下降及卵泡发育障碍[21],且高LH水平容易诱发早发LH峰[22],提示高比例多PN患者中,可能是偶遇高LH/FSH水平影响卵母细胞质量,同时也会使得促排卵过程中早发LH峰出现,影响卵母细胞成熟过程,最终对正常受精过程造成不利影响。也有研究发现PCOS患者LH/FSH比例过高,会导致妊娠率降低,流产率升高等不良妊娠结局的发生[23],与本文的结果一致。适当在促排卵治疗前对PCOS患者进行口服药物调整内分泌水平,使得LH/FSH水平降低,可能在一定程度上改善PCOS患者的卵母细胞质量,从而改善周期结局。
综上所述, PCOS患者多PN率高于输卵管因素不孕患者,与PCOS患者AMH、LH/FSH水平高有关。且PCOS患者中多PN率会导致囊胚形成率、生化妊娠率、活产率显著降低。如何降低PCOS患者的多PN率,提高卵子利用率,改善胚胎质量,仍需要后期扩大样本量进行进一步研究。
1 秦祖兴,唐永梅,牟联俊,等.IVF/ICSI周期中≥3PN胚胎用于囊胚培养方案优化的探讨.新医学,2019,50:295-297.
2 Grøndahl ML,Christiansen SL,Kesmodel US,et al.Effect of women′s age on embryo morphology,cleavage rate and competence-A multicenter cohort study.PLoS One,2017,12:e0172456.
3 Sayme N,Kljajic M,Krebs T,et al.The impact of anti-Müllerian hormone(AMH) on multiple pronuclei(PN) presence and oocyte maturity in ICSI treatments.Gynecol Endocrinol,2020,36:646-649.
4 di Clemente N,Racine C,Pierre A,et al.Anti-Müllerian hormone in female reproduction.Endocr Rev,2021,42:753-782.
5 张晗,贾婵维.PCOS不孕患者IVF-ET治疗及影响因素的研究.中国优生与遗传杂志,2023,31:427-430.
6 Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome(PCOS).Hum Reprod,2004,19:41-47.
7 Gardner DK,Lane M,Stevens J,et al.Blastocyst score affects implantation and pregnancy outcome:towards a single blastocyst transfer.Fertil Steril,2000,73:1155-1158.
8 Ortiz-Flores AE,Luque-Ramírez M,Escobar-Morreale HF.Polycystic ovary syndrome in adult women.Med Clin(Barc),2019,152:450-457.
9 李睿.二甲双胍联合炔雌醇环丙孕酮治疗多囊卵巢综合征不孕症的临床分析.北方药学,2018,15:61.
10 奚迪,郑英霞,刘斌,等.多囊卵巢综合征患者血清和卵泡液中Adropin水平对胚胎发育质量的影响.国际检验医学杂志,2019,40:1968-1972.
11 林碎玲,李雪梅,唐雪莲,等.体外受精形成多原核的影响因素及临床结局.中国妇幼保健,2017,32:2980-2983.
12 Macas E,Imthurn B,Keller PJ.Increased incidence of numerical chromosome abnormalities in spermatozoa injected into human oocytes by ICSI.Hum Reprod,2001,16:115-120.
13 Telfer EE,Andersen CY.In vitro growth and maturation of primordial follicles and immature oocytes.Fertil Steril,2021,115:1116-1125.
14 Santella L,Limatola N,Chun JT.Cellular and molecular aspects of oocyte maturation and fertilization:a perspective from the actin cytoskeleton.Zoological Lett,2020,6:5.
15 Chen M,He C,Zhu K,et al.Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication.Theranostics,2022,12:782-795.
16 Kim YJ,Ku SY,Jee BC,et al.Tri-pronucleated zygotes may occur less frequently in luteinizing hormone activity-added cycles.Gynecol Endocrinol,2011,27:458-463.
17 Li M,Zhao W,Xue X,et al.Three pro-nuclei(3PN) incidence factors and clinical outcomes:a retrospective study from the fresh embryo transfer of in vitro fertilization with donor sperm(IVF-D).Int J Clin Exp Med,2015,8:13997-14003.
18 Li M,Xue X,Zhang S,et al.Effects of triploidy incidence on clinical outcomes for IVF-ET cycles in different ovarian stimulation protocols.Gynecol Endocrinol,2015,31:769-773.
19 Chen W,Bai H,Li M,et al.Effects of three pro-nuclei(3PN) incidence on laboratory and clinical outcomes after early rescue intracytoplasmic sperm injection(rescue-ICSI):an analysis of a 5-year period.Gynecol Endocrinol,2021,37:137-140.
20 Pantos K,Sfakianoudis K,Maziotis E,et al.Abnormal fertilization in ICSI and its association with abnormal semen parameters:a retrospective observational study on 1855 cases.Asian J Androl,2021,23:376-385.
21 Shoham Z.The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation.Fertil Steril,2002,77:1170-1177.
22 Geng Y,Lai Q,Xun Y,et al.The effect of premature luteinizing hormone increases among high ovarian responders undergoing a gonadotropin-releasing hormone antagonist ovarian stimulation protocol.Int J Gynaecol Obstet,2018,142:97-103.
23 Neeta S,Neha M,Yogita D.Do basal luteinizing hormone and luteinizing hormone/follicle-stimulating hormone ratio have significance in prognosticating the outcome of In vitro Fertilization cycles in polycystic ovary syndrome?.J Hum Reprod Sci,2021,14:326.