妊娠糖尿病(gestational diabetes mellitus,GDM)是妊娠期最常见的代谢性紊乱疾病之一。GDM被定义为在妊娠期发病或首次发现的任何程度的葡萄糖耐受不良,但在妊娠前没有明显的糖尿病。Meta分析结果显示中国妇女的GDM患病率为13.4%(95%CI:10.6%~16.1%),其中35岁以下和35岁及以上的妇女患病率分别为10.8%和24.7%[1]。人群研究表明,GDM母亲在妊娠期及哺乳期暴露于高糖环境大大增加子代在儿童青少年时期出现肥胖症、代谢综合征[2]、糖耐量受损[3]等不良健康结局的风险。生命早期营养摄入在子代近、远期代谢健康中起到不可忽视的作用。
人乳低聚糖(human milk oligosaccharides,HMOs)是人乳中仅次于乳糖、脂质的第三大固体成分,公认为是母乳中关键的功能性生物分子,约占母乳总碳水化合物含量的20%[4]。分泌型母乳中HMOs主要由以下三种构成:岩藻糖基化的中性HMOs(35%~50%);唾液酸化的酸性HMOs(12%~14%);非岩藻糖基化的中性HMOs(42%~55%)[5]。HMOs含量受母亲健康状态影响。据报道,GDM母亲的初乳中HMOs含量显著低于健康母亲,然而成熟乳时HMOs含量却比健康母亲显著升高,且组间差异随着时间推移逐渐消失[6]。2′-岩藻糖基乳糖(2′-fucosyllactose,2′-FL)是分泌型母乳(约占妇女人群的70%~80%)中含量最高的HMOs[7]。是参与婴幼儿早期肠道微生物群构建的重要母乳成分。体外研究显示2′-FL可以促进婴儿肠道微生物群的发育和健康[8]。
本实验通过大鼠模型,探索2′-FL对GDM大鼠模型的仔鼠空腹血糖的影响。而后针对GDM大鼠模型的仔鼠,使用不同剂量的2′-FL在哺乳期进行灌胃干预,探索2′-FL是否对GDM子代空腹血糖受损具有改善作用。
材料与方法
一、实验材料
1. 试剂:2′-FL(纯度:90.7%,CAS:41263-94-9,Glycarbo公司)、链脲佐菌素(streptozocin,STZ)、柠檬酸钠溶液。
2. 设备和仪器:电子天平(SPN3001F,奥豪斯),电子天平(BS2000S,赛多利斯),气象色谱仪(GC-2030,岛津),质谱仪(QP202NX,岛津)。
3. 实验动物:健康成年SPF级雌性SD大鼠50只、雄鼠20只,由北京大学医学部试验动物科学部提供,实验动物生产许可证SCXK(京)2021-0013。动物饲养在屏障环境,温度(22±2)℃,相对湿度50%~60%,昼夜照明时间12 h:12 h。该实验此前已获得北京大学伦理委员会批准(LA2021107)。
二、实验方法
1. 实验动物造模:7周龄雌鼠按照体重随机分入空白对照组和GDM造模组,分别给予基础饲料和高脂饲料喂养4周。过夜禁食不禁水12 h后,采集尾静脉血,GDM组中将空腹血糖水平>6.7 mmol/L的雌性大鼠剔除。将雌鼠与雄鼠合笼交配,确认受孕的第5天早上对GDM造模组孕鼠腹腔注射1% STZ 30 mg/kg·BW,注射72 h后检测空腹血糖在6.7 mmol/L以上,即认为造模成功[9]。空白对照组孕鼠同一时间腹腔注射0.1 mol/L柠檬酸钠溶液。空白对照组孕鼠整个孕期进食基础饲料,GDM组孕鼠整个孕期继续进食高脂饲料并在哺乳期恢复正常饲料喂养。
2. 仔鼠2′-FL干预:将GDM造模组孕鼠随机分为对照组(GDM组)、GDM+2′-FL低剂量干预组(GLF组)、GDM+2′-FL中剂量干预组(GMF组)、GDM+2′-FL高剂量干预组(GHF组)。空白对照组保持不变。每组8只孕鼠。于产后第3~21天(PND3~21)对仔鼠(每组32对雌雄仔鼠)进行灌胃,空白对照组与GDM组灌胃蒸馏水,GLF组、GMF组和GHF组分别给予0.2 g/kg、0.6 g/kg和1.8 g/kg的2′-FL灌胃。所有仔鼠均正常饲料喂养。
3. 指标检测:各组母鼠在孕期和哺乳期每隔3 d监测体重;于孕第9天和PND3、PND11和PND21用快速血糖试纸测定空腹血糖水平;于PND2、PND10和PND20无菌采集乳汁,使用气相色谱-质谱联用法测定2′-FL含量。记录各窝分娩基本情况,PND3时随机留取各窝4对雌雄仔鼠。于PND7和PND21在各组随机选取8对雌雄仔鼠称量体重,处死后称量脑、胸腺、肝脏、脾脏、胰脏、肾脏重量。
4.统计学分析:实验结果以![]() 表示,使用SPSS 25.0软件进行统计分析。脏器系数(mg/g)=脏器重量(mg)/仔鼠体重(g)。采用单因素方差分析(one-way ANOVA),方差齐时,采用LSD法进行两两比较;方差不齐时,采用Tamhane′s法进行两两比较。P<0.05为差异具有统计学意义。
表示,使用SPSS 25.0软件进行统计分析。脏器系数(mg/g)=脏器重量(mg)/仔鼠体重(g)。采用单因素方差分析(one-way ANOVA),方差齐时,采用LSD法进行两两比较;方差不齐时,采用Tamhane′s法进行两两比较。P<0.05为差异具有统计学意义。
结 果
一、活产仔鼠基本情况
各组母鼠活产仔鼠情况如表1。各组孕鼠活产数为12~14只,平均出生体重为6~7 g。各组活产仔鼠数、平均出生体重及性别比差异均无统计学意义
表1 活产数仔鼠基本情况
Table 1 The basic characteristic of alive offspring(n=16)
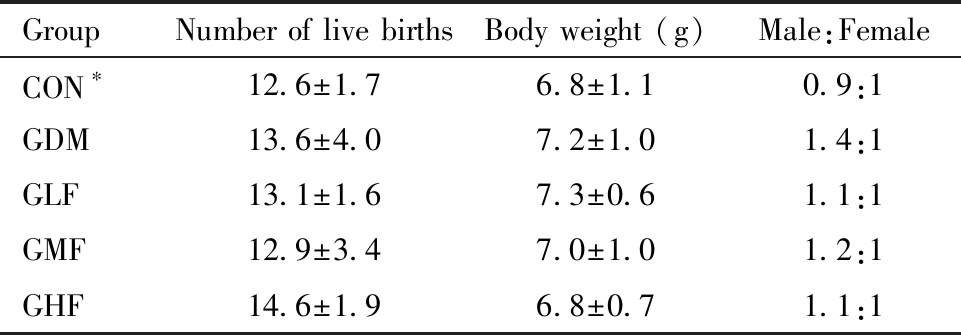
*CON:Control group
GroupNumber of live birthsBody weight (g)Male:FemaleCON∗12.6±1.76.8±1.10.9:1GDM13.6±4.07.2±1.01.4:1GLF13.1±1.67.3±0.61.1:1GMF12.9±3.47.0±1.01.2:1GHF14.6±1.96.8±0.71.1:1
二、母鼠体重、血糖及乳汁中2′-FL含量变化
母鼠孕期、哺乳期体重变化如图1所示,各组间差异无统计学意义。空腹血糖结果如图2所示,孕9天时所有GDM造模组均较空白对照组空腹血糖显著升高(P均<0.05),空白对照组空腹血糖为6~7 mmol/L,GDM模型组空腹血糖为10~14 mmol/L,提示GDM造模成功。哺乳期各组间母鼠空腹血糖差异无统计学意义,各组母鼠空腹血糖在5~7 mmol/L之间。如图3所示,PND3和PND21时GDM模型组乳汁中2′-FL含量显著低于空白组(P均<0.01)。所有孕鼠组的乳汁中2′-FL含量均随时间变化而显著减少(P=0.004)。而GDM模型组中2′-FL含量的降低较空白对照组有所延迟。
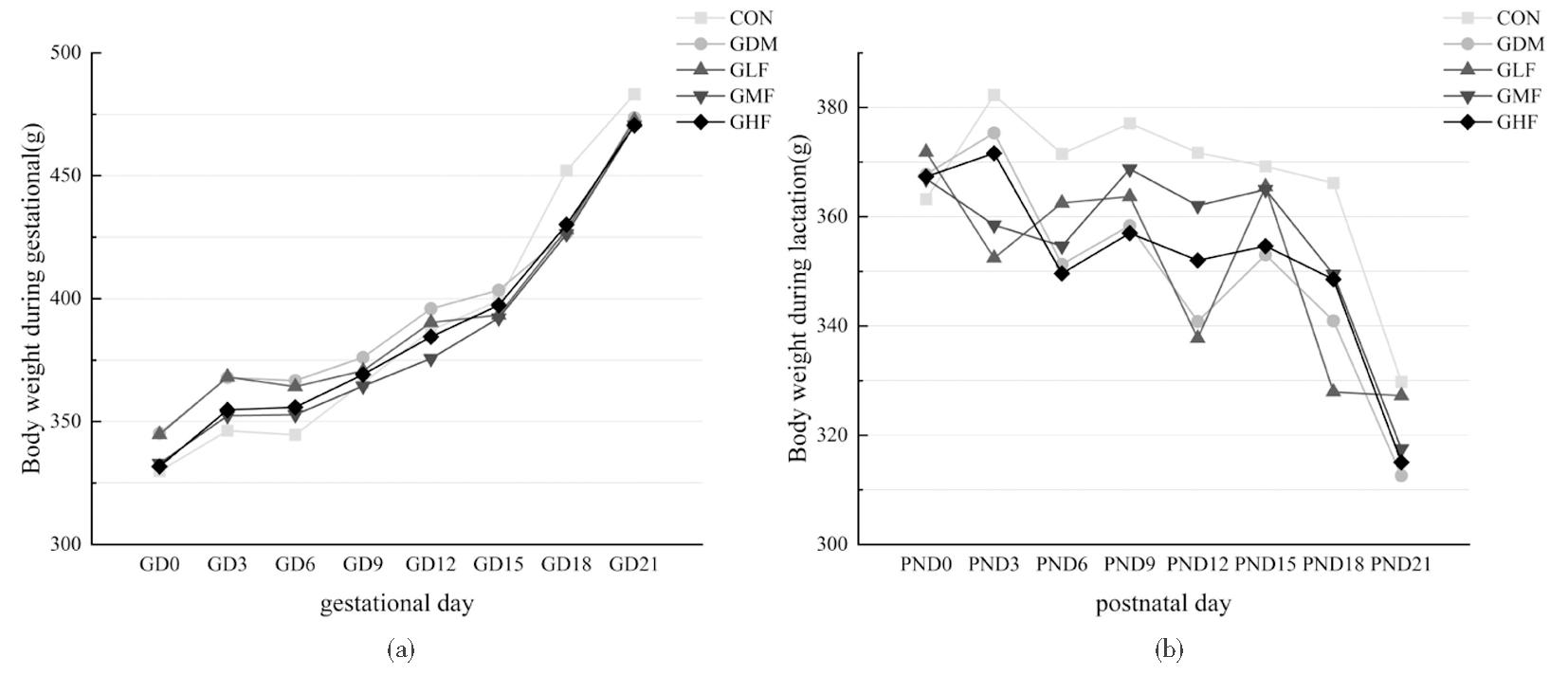
(a) Changes of body weight during gestation; (b) Changes of body weight during lactation. GD indicates pregnancy day; PND indicates postnatal day
图1 孕期及哺乳期母鼠体重变化
Figure 1 Changes of body weight during pregnancy and lactation
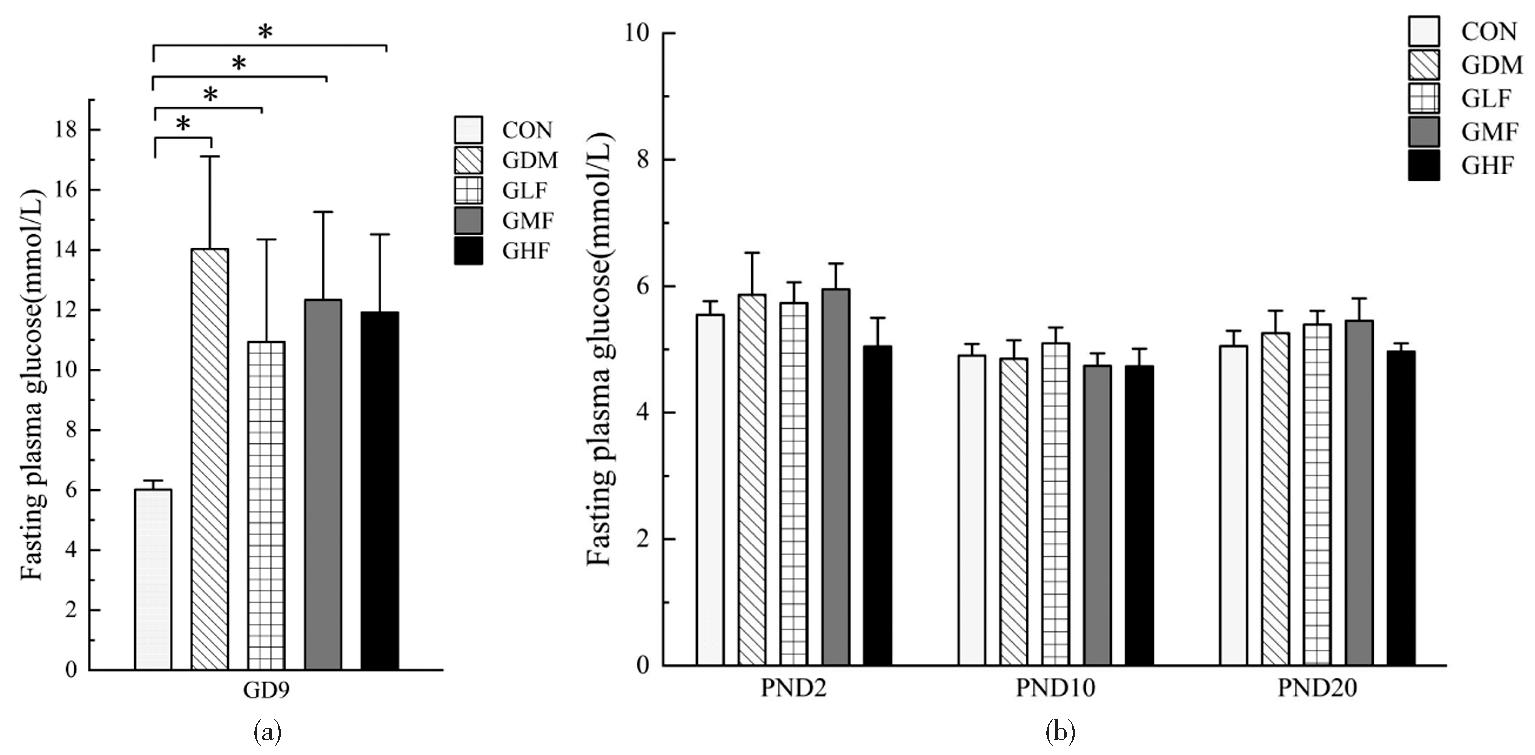
(a)FPG of maternal rats in GD9; (b) FPG of maternal rats in PND2、PND10 and PND20. compared with CON, *P<0.05
图2 孕期及哺乳期母鼠空腹血糖
Figure 2 Fasting plasma glucose of maternal rats during pregnancy and lactation
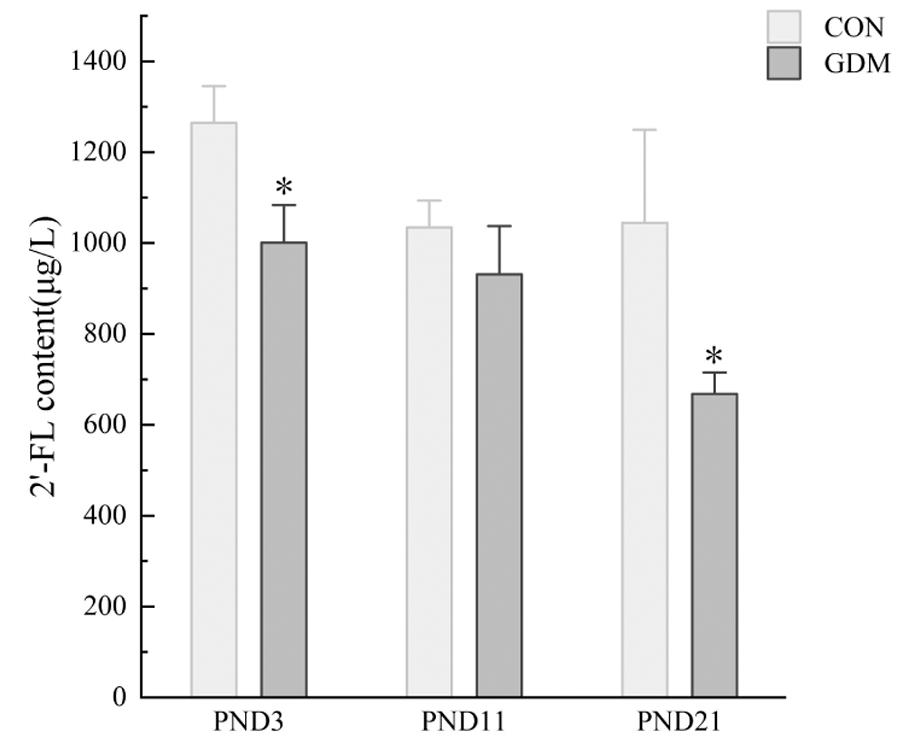
Compared with CON, *P<0.05
图3 母鼠乳汁2′-FL变化
Figure 3 The change of 2′-FL content during lactation
三、仔鼠体重及脏器系数变化
PND7时仔鼠体重及脏器系数如表2,雄性仔鼠GDM组肝脏系数显著低于空白对照组(P=0.009)。雌性仔鼠GDM组肝脏系数显著低于空白对照组(P=0.001),GHF组肝脏系数显著高于GDM组(P=0.014)。
表2 PND7时仔鼠体重及脑、胸腺、肝脏、脾脏、胰脏和肾脏的脏器系数
Table 2 The weight and indexes of brain, thymus, liver, spleen, pancreas and kidney among offspring rats in PND7(n=8)
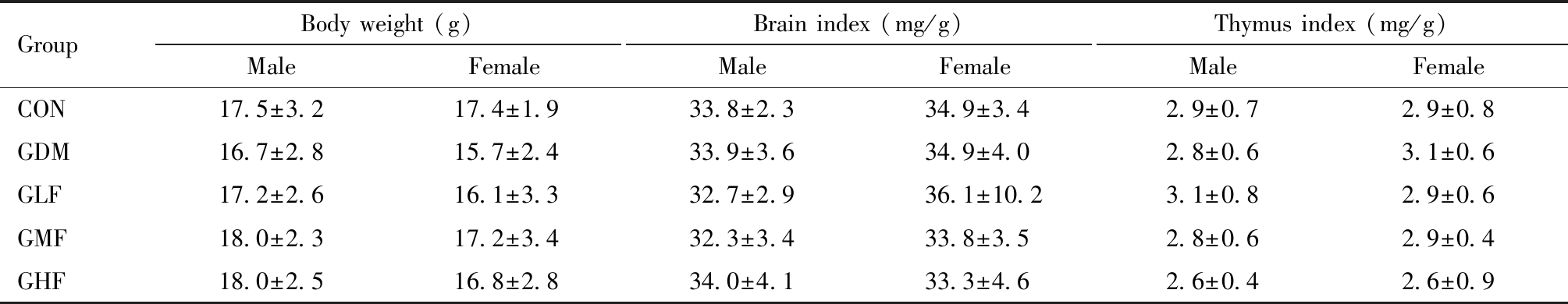
GroupBody weight (g)MaleFemaleBrain index (mg/g)MaleFemaleThymus index (mg/g)MaleFemaleCON17.5±3.217.4±1.933.8±2.334.9±3.42.9±0.72.9±0.8GDM16.7±2.815.7±2.433.9±3.634.9±4.02.8±0.63.1±0.6GLF17.2±2.616.1±3.332.7±2.936.1±10.23.1±0.82.9±0.6GMF18.0±2.317.2±3.432.3±3.433.8±3.52.8±0.62.9±0.4GHF18.0±2.516.8±2.834.0±4.133.3±4.62.6±0.42.6±0.9
表2(续)
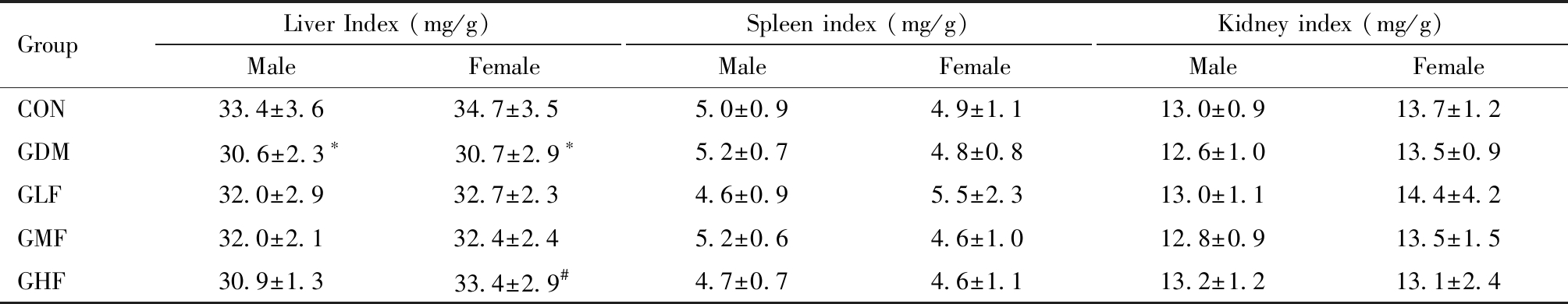
Compared with CON, *P<0.05; compared with GDM, #P<0.05
GroupLiver Index (mg/g)MaleFemaleSpleen index (mg/g)MaleFemaleKidney index (mg/g)MaleFemaleCON33.4±3.634.7±3.55.0±0.94.9±1.113.0±0.913.7±1.2GDM30.6±2.3∗30.7±2.9∗5.2±0.74.8±0.812.6±1.013.5±0.9GLF32.0±2.932.7±2.34.6±0.95.5±2.313.0±1.114.4±4.2GMF32.0±2.132.4±2.45.2±0.64.6±1.012.8±0.913.5±1.5GHF30.9±1.333.4±2.9#4.7±0.74.6±1.113.2±1.213.1±2.4
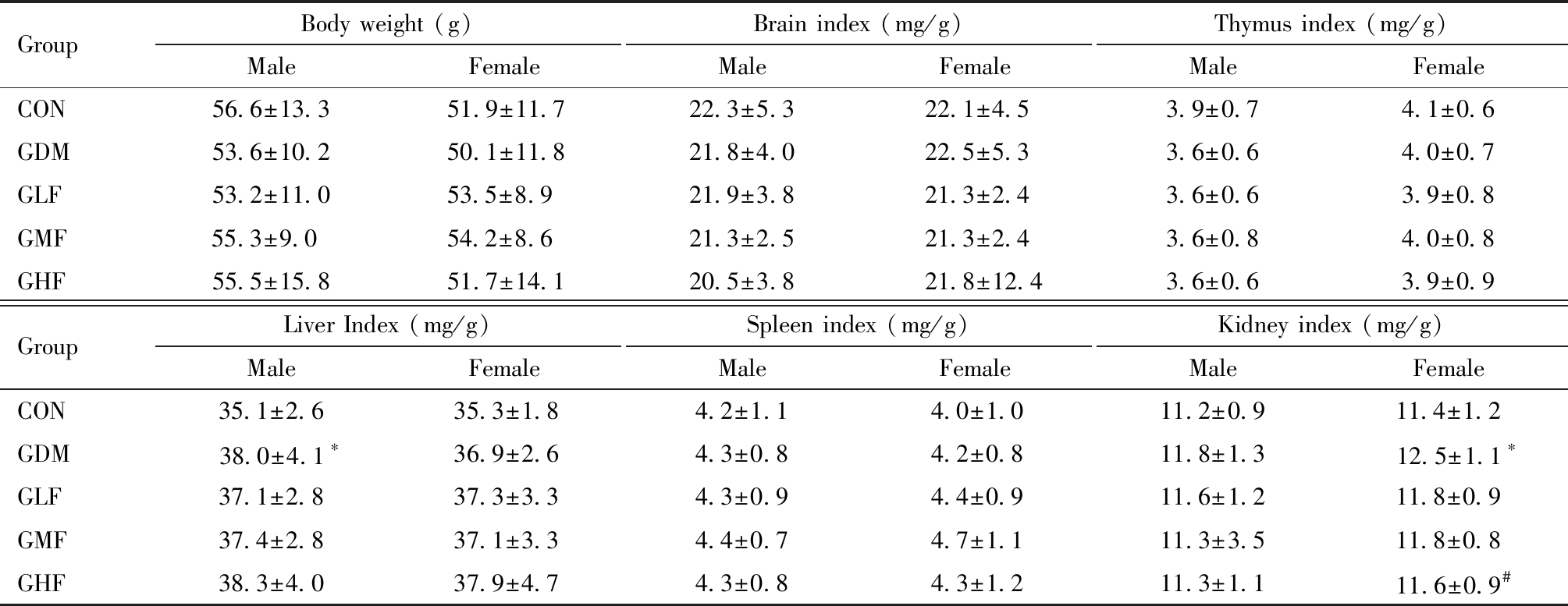
PND21结果如表3,雄性仔鼠GDM组肝脏系数显著高于空白对照组(P=0.046)。雌性仔鼠GDM组肾脏系数显著高于空白对照组(P=0.010),GHF组肾脏系数显著低于GDM组(P=0.031)。
表3 PND21时仔鼠体重及脑、胸腺、肝脏、脾脏、胰脏和肾脏的脏器系数
Table 3 The weight and indexes of brain, thymus, liver, spleen, pancreas and kidney among offspring rats in PND21(n=8)
Compared with CON,*P<0.05; compared with GDM, #P<0.05
GroupBody weight (g)MaleFemaleBrain index (mg/g)MaleFemaleThymus index (mg/g)MaleFemaleCON56.6±13.351.9±11.722.3±5.322.1±4.53.9±0.74.1±0.6GDM53.6±10.250.1±11.821.8±4.022.5±5.33.6±0.64.0±0.7GLF53.2±11.053.5±8.9 21.9±3.821.3±2.43.6±0.63.9±0.8GMF55.3±9.0 54.2±8.6 21.3±2.521.3±2.43.6±0.84.0±0.8GHF55.5±15.851.7±14.120.5±3.821.8±12.43.6±0.63.9±0.9GroupLiver Index (mg/g)MaleFemaleSpleen index (mg/g)MaleFemaleKidney index (mg/g)MaleFemaleCON35.1±2.635.3±1.84.2±1.14.0±1.011.2±0.911.4±1.2GDM38.0±4.1∗36.9±2.64.3±0.84.2±0.811.8±1.312.5±1.1∗GLF37.1±2.837.3±3.34.3±0.94.4±0.911.6±1.211.8±0.9GMF37.4±2.837.1±3.34.4±0.74.7±1.111.3±3.511.8±0.8GHF38.3±4.037.9±4.74.3±0.84.3±1.211.3±1.111.6±0.9#
四、仔鼠空腹血糖
仔鼠于PND21检测空腹血糖,当不考虑性别时结果如图4(a)所示:GDM组显著高于空白对照组(P<0.001),GLF组、GMF组和GHF组空腹血糖显著低于GDM组(P均<0.01)。考虑性别时如图4(b)所示:雌性仔鼠GDM组空腹血糖显著高于空白对照组(P=0.002),GLF组空腹血糖显著低于GDM组(P=0.010)。雄性仔鼠GDM组空腹血糖显著高于空白对照组(P<0.001),GLF组、GMF组和GHF组空腹血糖显著低于GDM组(P均<0.05)。
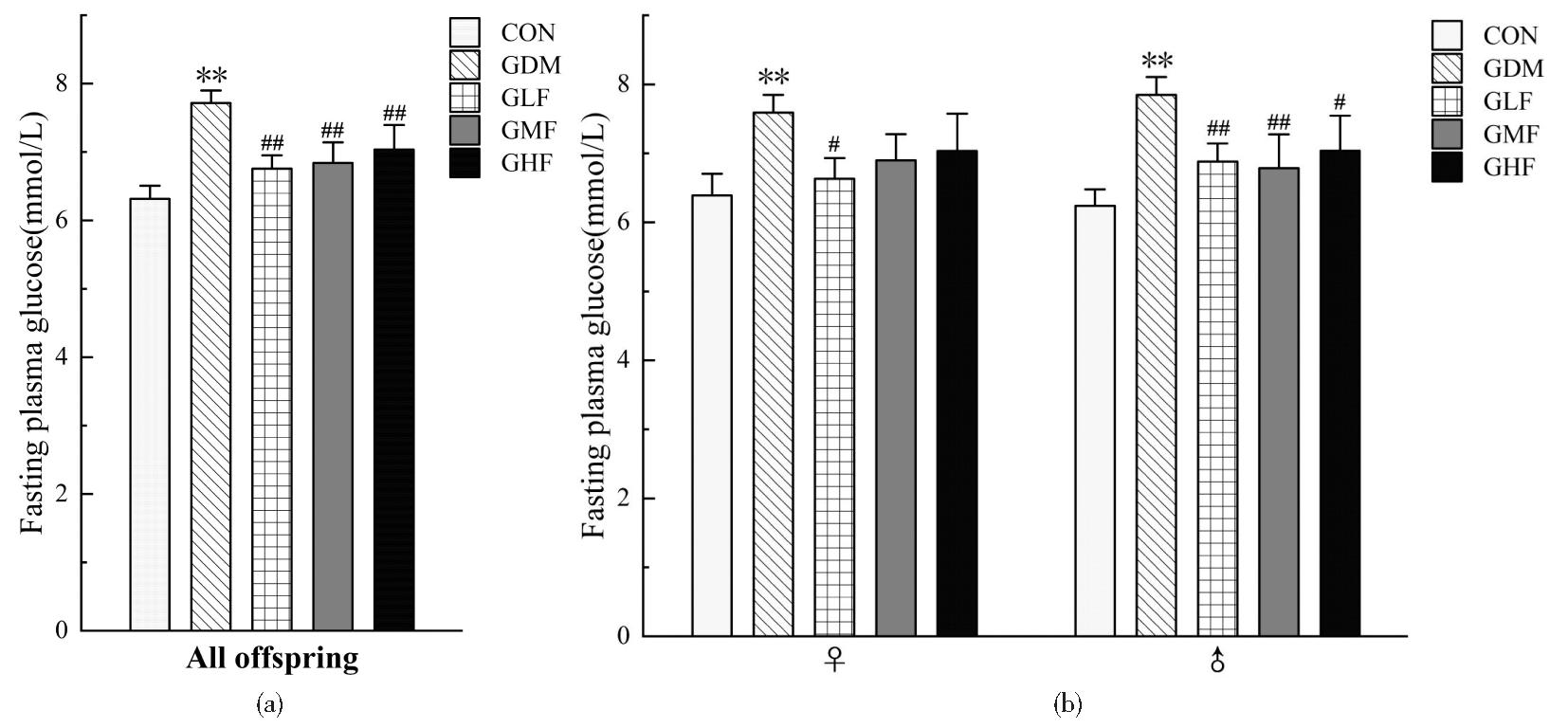
(a) FPG of all offspring; (b) FPG of female and male offspring separately. Compared with CON, **P<0.01; compared with GDM, #P<0.05, ##P<0.01
图4 PND21时仔鼠空腹血糖
Figure 4 Fasting plasma glucose of offspring in PND21
讨 论
本研究通过建立GDM大鼠模型,探索2′-FL在GDM大鼠乳汁中不同时期的含量变化。而后针对GDM大鼠子代,使用不同剂量的2′-FL在哺乳期进行灌胃干预,探索2′-FL是否对GDM大鼠子代的糖代谢异常具有改善作用。根据欧盟食品安全局建议,2′-FL干预剂量为1.2 g/L[10]。根据《中国居民膳食营养参考摄入量》,以6月龄婴儿每日摄入700 mL母乳为基准,体重8 kg的婴儿单日2′-FL干预剂量计算结果为0.1 g/kg。按体表面积折算的等效剂量比值,大鼠的等效剂量约为人的6.25倍,因此换算到大鼠的干预剂量约为0.6 g/kg。本实验以0.6 g/kg为中间剂量设置剂量梯度。实验结果显示,产后第3天和第21天(分别对应初乳和成熟乳)时GDM组乳汁中2′-FL含量较空白对照组显著降低,两组乳汁中2′-FL含量均随时间递减。空白对照组中2′-FL含量在过渡乳时期显著降低随后维持,GDM组则于成熟乳时才出现2′-FL含量骤降的现象。GDM雌、雄仔鼠产后第21天的空腹血糖较空白对照组显著升高,GLF组雌雄仔鼠空腹血糖较GDM组显著降低,其余干预剂量组仅在雄性仔鼠中产生改善空腹血糖受损作用。产后第7天GDM组雌雄性仔鼠肝脏系数较空白对照组显著降低,GHF组雌性仔鼠肝脏系数较GDM组显著升高。产后第21天GDM组雄性仔鼠肝脏系数较空白对照组显著升高。GDM组雌性仔鼠肾脏系数较空白对照组显著升高,GHF组较GDM组显著降低。
GDM对母亲乳汁HMOs含量有所影响,本研究发现GDM组初乳、成熟乳2′-FL含量较空白对照组显著减少,各组2′-FL含量均在哺乳期内随时间不断减少。Soyy lmaz等[11]总结大量文献得出人乳中2′-FL含量从初乳到成熟乳逐渐降低。GDM与母体代谢息息相关,有研究显示HMOs自妊娠早期就存在与母体循环中,并发现唾液酸化HMOs与空腹血糖相关[12]。而直接关注GDM与人乳HMOs关系的研究很少,2022年Wang等[13]发现中国西北地区患有GDM的母亲乳汁中唾液酸低聚糖水平的降低。McGuire等[14]和M Tonon等[15]的观察性研究均发现母乳2′-FL含量与母亲体重和BMI呈负相关关系。母体的代谢紊乱很可能是导致乳汁成分变化的重要因素。
lmaz等[11]总结大量文献得出人乳中2′-FL含量从初乳到成熟乳逐渐降低。GDM与母体代谢息息相关,有研究显示HMOs自妊娠早期就存在与母体循环中,并发现唾液酸化HMOs与空腹血糖相关[12]。而直接关注GDM与人乳HMOs关系的研究很少,2022年Wang等[13]发现中国西北地区患有GDM的母亲乳汁中唾液酸低聚糖水平的降低。McGuire等[14]和M Tonon等[15]的观察性研究均发现母乳2′-FL含量与母亲体重和BMI呈负相关关系。母体的代谢紊乱很可能是导致乳汁成分变化的重要因素。
母体妊娠期间的健康状态无疑是仔鼠健康状态的直接影响因素之一。本研究发现GDM子代在生命早期即出现空腹血糖受损(impaired glucose tolerance,IGF),这一现象与大量人群队列研究结论相同[3, 16]。上世纪五十年代就有学者提出母亲妊娠期间体内过量的葡萄糖很可能通过胎盘进入胎儿,导致高胰岛素血症和脂肪的过量堆积[17]。类似的“胎儿编程”假说认为生命早期关键发育窗口的营养暴露可能引起生长发育的变化从而导致远期慢性疾病风险的增加[18]。因此在生命早期及时对仔鼠进行干预至关重要。本研究采用不同剂量2′-FL在哺乳期对GDM仔鼠进行干预,观察其空腹血糖、体重以及主要脏器系数。本研究观察到2′-FL干预后GDM仔鼠糖代谢紊乱的改善现象。2′-FL作为HMOs其中的一种,能够躲避婴儿胃肠的消化到达远端肠道发挥益生元的作用[19]。使用2′-FL干预无宿主人类结肠模型的研究发现其可显著增加结肠乙酸、丁酸和丙酸的产生[8]。人群研究显示补充2′-FL可显著增加糖尿病前期男性循环内的丁酸盐浓度[20]。本研究中2′-FL很可能是通过富集了产生短链脂肪酸的肠道微生物,增加了循环短链脂肪酸水平,从而对葡萄糖稳态的调节起到积极作用。
通过对产后两个时间点的脏器系数比较得出,GDM主要影响仔鼠肝脏和肾脏系数,而2′-FL高剂量干预可逆转GDM雌性仔鼠的这一变化。产后第7天的脏器系数显示GDM组仔鼠肝脏系数显著低于空白对照组,经2′-FL高剂量干预后雌性仔鼠肝脏系数较GDM组显著升高。这一结果与Yao等[21]的研究相似,他们使用2′-FL治疗结肠炎小鼠后发现治疗组的肝脏重量较结肠炎小鼠显著增加了12.65%。产后第21天GDM组雄性仔鼠的肝脏系数和雌性仔鼠的肾脏系数较空白对照组显著升高,2′-FL高剂量干预组雌性仔鼠肾脏系数较GDM组显著降低。来自北京市的队列研究发现GDM仔鼠出生后0~3月龄见出现体重增长缓慢现象,随后速度加快追赶上正常儿童[22]。GDM子代内脏系数在哺乳期终止时的升高是否可能与追赶生长有关,需要进一步的探索。
综上,GDM可影响仔鼠产后第21天空腹血糖,2′-FL哺乳期干预可一定程度改善仔鼠的空腹血糖受损情况,在本研究对仔鼠使用三个2′-FL剂量进行干预后发现2′-FL低剂量干预组可以同时改善雌、雄仔鼠糖代谢异常。考虑到2′-FL的肠道微生态改善作用,进一步机制有待再研究。2′-FL中、高剂量的干预对于雌性仔鼠糖代谢异常无显著改善效果可能与GDM对雌性子代有更顽固的影响有关。但具体机制及适宜剂量还需进一步研究。本研究成果为孕期及哺乳期暴露于高糖环境的子代生命早期的膳食干预提供了思路。
1 武亚星,姚晓燕,周立芳,等.中国2012-2020年妊娠期糖尿病患病率的Meta分析.现代医学,2023,51:879-884.
2 Tam WH,Ma RC,Yang X,et al.Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero.Pediatrics,2008,122:1229-1234.
3 Lowe WL Jr,Scholtens DM,Kuang A,et al.Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS):Maternal gestational diabetes mellitus and childhood glucose metabolism.Diabetes Care,2019,42:372-380.
4 Urashima T,Asakuma S,Leo F,et al.The predominance of type I oligosaccharides is a feature specific to human breast milk.Adv Nutr,2012,3:473S-82S.
5 Dinleyici M,Barbieur J,Dinleyici EC,et al.Functional effects of human milk oligosaccharides (HMOs).Gut Microbes,2023,15:2186115.
6 Li X,Ning X,Rui B,et al.Alterations of milk oligosaccharides in mothers with gestational diabetes mellitus impede colonization of beneficial bacteria and development of RORγt+ Treg cell-mediated immune tolerance in neonates.Gut Microbes,2023,15:2256749.
7 Liu S,Cai X,Wang J,et al.Six oligosaccharides′ variation in breast milk:A study in south China from 0 to 400 days postpartum.Nutrients,2021,13:4017.
8 Zhang S,Chen L,Hu M,et al.2′-Fucosyllactose (2′-FL) changes infants gut microbiota composition and their metabolism in a host-free human colonic model.Food Res Int,2023,173:113293.
9 Zhou Y,Zhao R,Lyu Y,et al.Serum and amniotic fluid metabolic profile changes in response to gestational diabetes mellitus and the association with maternal-fetal outcomes.Nutrients,2021,13:3644.
10 EFSA Panel on Nutrition,NFaFA(DNA),Turck D,et al.Safety of the extension of use of 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) as novel foods in food supplements for infants pursuant to Regulation (EU) 2015/2283.EFSA J,2022,20:e07257.
11 Soyy lmaz B,Mikš MH,Röhrig CH,et al.The mean of milk:A review of human milk oligosaccharide concentrations throughout lactation.Nutrients,2021,13:2737.
lmaz B,Mikš MH,Röhrig CH,et al.The mean of milk:A review of human milk oligosaccharide concentrations throughout lactation.Nutrients,2021,13:2737.
12 Jantscher-Krenn E,Treichler C,Brandl W,et al.The association of human milk oligosaccharides with glucose metabolism in overweight and obese pregnant women.Am J Clin Nutr,2019,110:1335-1343.
13 Wang X,Liu J,Li C,et al.Pregnancy-related diseases and delivery mode can affect the content of human milk oligosaccharides:A preliminary study.J Agric Food Chem,2022,70:5207-5217.
14 McGuire MK,Meehan CL,McGuire MA,et al.What′s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically.Am J Clin Nutr,2017,105:1086-1100.
15 M Tonon K,B de Morais M,F V Abrão AC,et al.Maternal and infant factors associated with human milk oligosaccharides concentrations according to secretor and lewis phenotypes.Nutrients,2019,11:1358.
16 Brown FM,Isganaitis E,James-Todd T.Much to HAPO FUS about:increasing maternal glycemia in pregnancy is associated with worsening childhood glucose metabolism.Diabetes Care,2019,42:393-395.
17 PEDERSEN J.Weight and length at birth of infants of diabetic mothers.Acta Endocrinol (Copenh),1954,16:330-342.
18 Barker DJ,Osmond C.Infant mortality,childhood nutrition,and ischaemic heart disease in England and Wales.Lancet,1986,1:1077-1081.
19 Engfer MB,Stahl B,Finke B,et al.Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract.Am J Clin Nutr,2000,71:1589-1596.
20 Canfora EE,Vliex L,Wang T,et al.2′-fucosyllactose alone or combined with resistant starch increases circulating short-chain fatty acids in lean men and men with prediabetes and obesity.Front Nutr,2023,10:1200645.
21 Yao Q,Gao Y,Fan L,et al.2′-Fucosyllactose remits colitis-induced liver oxygen stress through the gut-liver-metabolites axis.Nutrients,2022,14:4186.
22 侯杉杉,杨洁.妊娠糖尿病母亲子代0~36月龄生长发育情况研究.中国儿童保健杂志,2019,27:775-778.