宫颈癌(cervical cancer,CC)是常见的女性恶性肿瘤,全球每年有超过50万例新增病例[1]。宫颈上皮内瘤变(cervical intraepithelial neoplasia,CIN)是CC进展过程中的必经阶段,CIN可分为低级别与高级别,其中宫颈高级别上皮内瘤变(high-grade squamous intraepithelial lesion,HSIL)较为多见,虽未到达癌症的程度,但已累及宫颈组织较深位置[2]。CIN的发展和最终导致侵袭性癌症的主要原因是人乳头状瘤病毒(human papilloma virus,HPV)的持续感染[3-4]。宫颈环形电切术(loop electrosurgical excision procedure,LEEP)是CIN临床治疗的常用手术方法,能切除病变组织并清除HPV,降低HSIL进一步癌变风险。但仍有部分CIN患者在LEEP手术后HPV持续感染,大幅增加了CC患病风险,严重危害患者生命健康,因此研究CIN患者LEEP术后HPV持续感染高危因素具有重要意义[5]。近期有研究表明,人体受到病毒感染会引起T淋巴细胞减少,损害细胞免疫功能呢,导致机体免疫应答水平降低,最终诱发持续感染和肿瘤的发生[6-7]。并且HSIL患者术后容易出现脱痂、出血的情况,难以完全清除病毒感染病灶,残留HPV感染的清除还需依靠患者自身免疫力[8]。因此本文将探讨机体免疫应答与HSIL患者术后HPV转归的关系,现报道如下。
对象与方法
一、研究对象
选择本院在2020年9月—2022年3月期间收治的213例HSIL患者为研究对象,年龄为28~63岁,平均年龄(39.0±4.8)岁,纳入标准:(1)明确诊断为HSIL[9];(2)至少有2年性生活史;(3)HPV感染者;(4)术前48 h内无性生活、阴道冲洗、放药;(5)近3个月内未进行阴道相关疾病治疗;(6)治疗方法为LEEP刀宫颈锥切术。排除标准:(1)严重肾功能异常者;(2)恶性肿瘤患者;(3)严重心功能异常者;(4)妊娠或哺乳期妇女。本研究治疗方案已通过本院医学伦理委员会批准。
二、研究方法
1.治疗方法:213例HSIL患者均接受LEEP刀宫颈锥切术治疗。术前进行常规检查,包括B超检查、白带常规、阴道镜检查等,告知患者术前24 h禁止性生活,术前排空膀胱。术中,指导患者取膀胱截石位,行常规消毒后进行宫颈碘试验,标记碘溶液在宫颈表面移行区范围外5 mm左右,明确病变范围,利用高频电波LEEP刀切割病变组织,将功率设定为50 W,切割深度5~10 mm,若病灶面积大,可进行分次切除,利用球形电极电凝止血,涂抹烧伤湿润膏,利用碘伏纱布压迫填塞。术后,采用抗生素处理预防感染。
2.一般资料:收集患者的基本资料用于本次研究,其中包括患者年龄、月经规律、经期同房、饮酒、主动或被动吸烟、孕次、产次、病程、首次性生活年龄、宫颈癌家族史及流产史等资料。
3.临床指标检测:分别在患者入院、术后3月、术后6月及术后12月时抽取肘静脉血两管各2 mL,一管抗凝处理后,利用流式细胞仪检测患者T淋巴细胞亚群分布情况,包括CD3+、CD4+、CD8+细胞绝对数,CD4+/CD8+计算值为辅助性T细胞(helper T cell,Th)与抑制性T细胞(suppressor T cell,Ts)比值(Th/Ts);另取一管采用免疫比浊法检测免疫球蛋白G(immunoglobulin G,IgG)、免疫球蛋白M(immunoglobulin M,IgM)及免疫球蛋白A(immunoglobulin A,IgA)水平。
患者入院次日清晨采集空腹血液样本5 mL,离心15 min(3 000 rpm/min,离心半径10 cm)分离血清,全自动生化分析仪以酶联免疫吸附法测定肿瘤坏死因子-α(tumor necrosis factor,TNF-α)和白介素-6(interleukin-6,IL-6)水平。
4.阴道微环境检测:患者入院时进行样本采集,于阴道上1/3段无菌棉签旋转10 s取材,并立即送检。镜检阴道乳酸菌、滴虫、线索细胞及其他病原微生物等。阴道微环境检测[10]:(1)采用五联检测试剂盒检测白细胞脂酶(leukocyte esterase,LE)、过氧化氢(H2O2)、唾液酸苷酶(Sialidase,SIA)及pH值。(2)乳酸杆菌:显微镜下计数乳酸杆菌,乳酸杆菌+或++++为阳性,++和+++为阴性。(3)阴道清洁度(Cleanliness,CLE):镜检分泌物以杆菌为主,并且可见大量上皮细胞为Ⅰ度;有部分杆菌、脓细胞及杂菌,并且可见上皮细胞为Ⅱ度;有大量脓细胞和杂菌,少量杆菌和上皮细胞为Ⅲ度;几乎全布是脓细胞和杂菌为Ⅳ度。Ⅰ~Ⅱ度为CLE正常,Ⅲ~Ⅳ度为CLE异常。(4)患者Nugent评分超过6分并且线索细胞比例超过20%诊断为细菌性阴道病(bacterial vaginosis,BV);镜检发现阴道毛滴虫诊断为滴虫性阴道炎(trichomonas vaginitis,TV);镜检发现芽生孢子或菌丝诊断为外阴阴道假丝酵母菌病(vulvovaginal candidiasis,VVC)。
5.HPV检测:患者入院和术后12月时进行HPV检测,使用阴道窥器充分暴露宫颈,宫颈刷采集宫颈脱落上皮细胞样本,将宫颈刷浸入细胞保存液中,检测时将该样本离心1 min(转速10 000 r/min,半径3 cm)弃上清液,加入裂解酶液,提取DNA。
溶液配置:PCRmix 23.25 μL+DNAtaq酶 0.75 μL+1 μL模板。
反应条件:95 ℃预变性5 min;95 ℃变性1 min、55 ℃退火30 s、72 ℃延伸30 s,共40个循环;72 ℃终延伸5 min。
导流杂交:(1)取PCR产物20 μL,95 ℃加热5 min,冰水浴至少2 min;(2)1 μL杂交液预热45 ℃,温育至少2 min后;(3)将DNA样品溶液加入0.5 ml预热至45 ℃的杂交液,温育10 min后,进行导流杂交;使用0.8 mL杂交液反复冲洗膜三次;(4)杂交仪设定25 ℃,加入0.5 mL酶标液温育4 min开泵,再设定温度36 ℃彻底洗膜4次,加入NBT/BCIP溶液0.5 mL,盖上盖板显色3~6 min;(5)阳性点为蓝紫色远点,根据HPV分型比对图(图1)判断HPV分型。
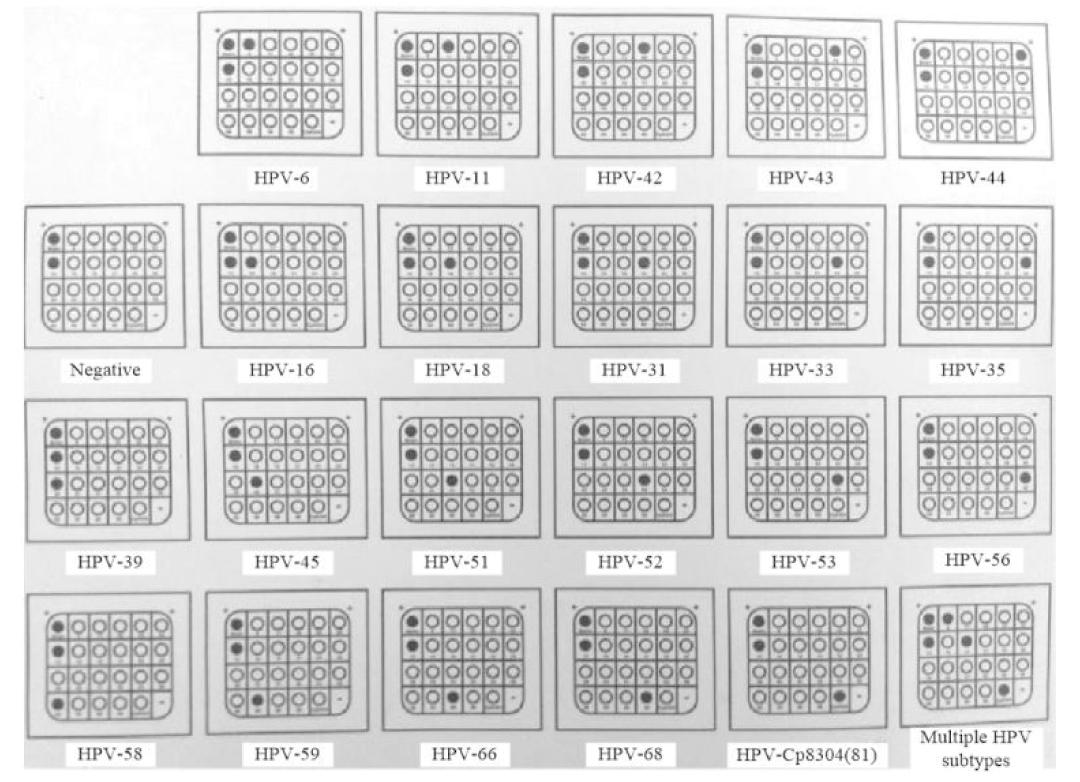
图1 HPV分型比对图
Figure 1 Comparison of HPV typing
三、统计学分析
应用SPSS 22.0软件进行数据分析,研究中计量资料和计数资料分别用均数±标准差![]() 和例数(%)表示,组间比较采用χ2检验、t检验。采用多因素Logistic回归分析HSIL患者术后HPV转归情况的影响因素,比较各因素对HPV转归的预测效能,绘制受试者工作特征(receiver operating characteristic,ROC)曲线,计算曲线下面积(area under curve,AUC)、灵敏度与特异度。Logistic模型调整混杂变量后,分析影响因素对HSIL患者术后HPV转归的关联性。
和例数(%)表示,组间比较采用χ2检验、t检验。采用多因素Logistic回归分析HSIL患者术后HPV转归情况的影响因素,比较各因素对HPV转归的预测效能,绘制受试者工作特征(receiver operating characteristic,ROC)曲线,计算曲线下面积(area under curve,AUC)、灵敏度与特异度。Logistic模型调整混杂变量后,分析影响因素对HSIL患者术后HPV转归的关联性。
结 果
一、HSIL患者手术前后T淋巴细胞亚群水平比较
比较213例HSIL患者手术前后T淋巴细胞亚群水平发现,术后3月、6月及12月患者CD3+、CD4+细胞数和Th/Ts水平与术前比较显著升高,CD8+细胞数显著降低(P均<0.05),见表1。
表1 HSIL患者术前、术后3月、术后6月及术后12月T淋巴细胞亚群水平比较![]()
Table 1 Comparison of T lymphocyte subsets in patients with HSIL before surgery, 3 months after surgery, 6 months after surgery and 12 months after surgery ![]()

Note:Compared with pre-operation,aP<0.05; Compared with 3 months after operation, bP<0.05; Compared with 6 months after operation, cP<0.05
ItemsPre-operation3 months after operation6 months after operation12 months after operationCD3+48.2±4.252.7±4.7a55.6±4.4ab57.2±4.4abCD4+20.3±3.022.6±2.5a24.8±2.6ab25.2±2.3abCD8+35.7±3.933.7±3.5a31.4±3.2ab28.6±3.4abcTh/Ts0.6±0.20.7±0.1a0.8±0.2ab0.9±0.2abc
二、HSIL患者手术前后免疫功能比较
术后HSIL患者IgA、IgG及IgM水平均显著降低,差异具有统计学意义(P均<0.05),随着术后时间的增加,IgA、IgG及IgM水平均呈下降趋势,见表2。
表2 HSIL患者术前、术后3月、术后6月及术后12月免疫功能比较![]()
Table 2 Comparison of immune function of HSIL patients before surgery, 3 months after surgery, 6 months after surgery and 12 months after ![]()
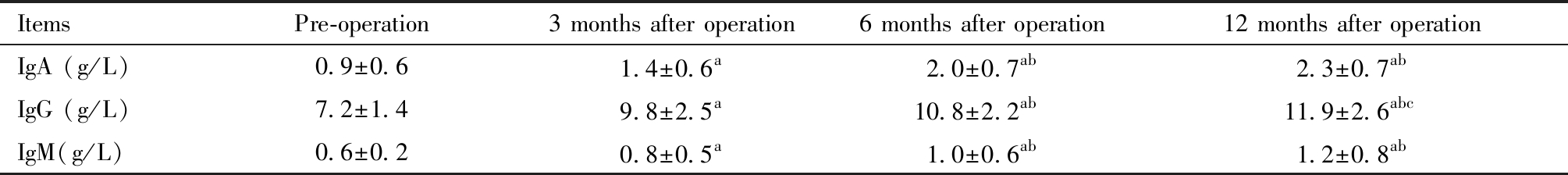
Note:Compared with pre-operation,aP<0.05; Compared with 3 months after operation, bP<0.05; Compared with 6 months after operation, cP<0.05
ItemsPre-operation3 months after operation6 months after operation12 months after operationIgA (g/L)0.9±0.61.4±0.6a2.0±0.7ab2.3±0.7abIgG (g/L)7.2±1.49.8±2.5a10.8±2.2ab11.9±2.6abcIgM(g/L)0.6±0.20.8±0.5a1.0±0.6ab1.2±0.8ab
三、两组患者一般资料比较
对转阴组和未转阴组患者一般资料进行比较,发现饮酒、主动或被动吸烟、月经规律、经期同房、首次性生活年龄、孕次、产次、宫颈癌家族史、病程及是否HPV16/18感染对HSIL患者术后HPV转归无显著影响(P均>0.05),未转阴组患者年龄显著高于转阴组,且未转阴组患者有流产史的比例显著高于转阴组(P均<0.05),见表3。
表3 转阴组和未转阴组患者一般资料比较[例(%)]
Table 3 Comparison of general data of patients in the negative conversion group and the non-negative conversion group[n(%)]
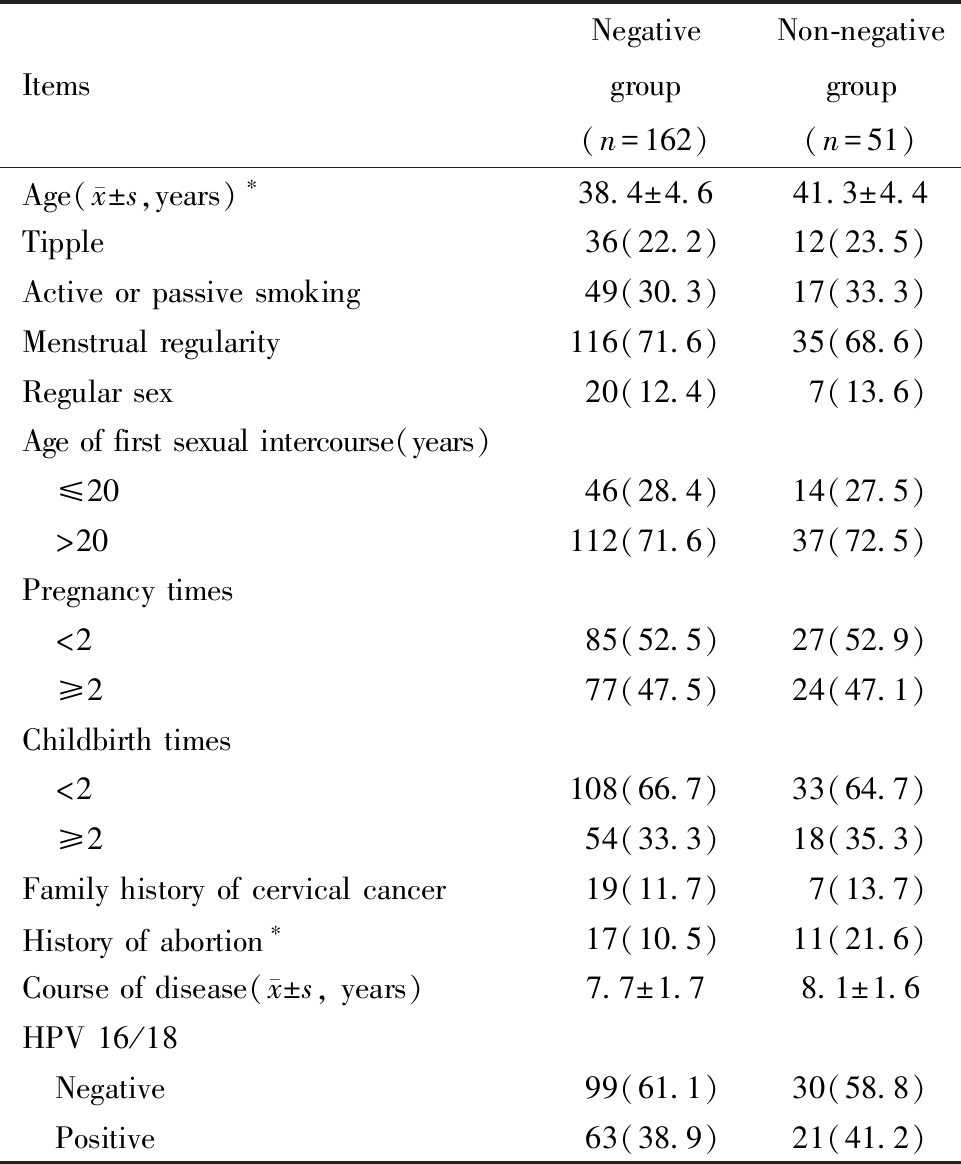
Note:Comparison betweenthe two groups,*P<0.05
ItemsNegative group(n=162)Non-negative group(n=51)Age( x±s,years)∗38.4±4.641.3±4.4Tipple36(22.2)12(23.5)Active or passive smoking49(30.3)17(33.3)Menstrual regularity116(71.6)35(68.6)Regular sex20(12.4)7(13.6)Age of first sexual intercourse(years) ≤2046(28.4)14(27.5) >20112(71.6)37(72.5)Pregnancy times <285(52.5)27(52.9) ≥277(47.5)24(47.1)Childbirth times <2108(66.7)33(64.7) ≥254(33.3)18(35.3)Family history of cervical cancer19(11.7)7(13.7)History of abortion∗17(10.5)11(21.6)Course of disease( x±s, years)7.7±1.78.1±1.6HPV 16/18 Negative99(61.1)30(58.8) Positive63(38.9)21(41.2)
四、两组患者临床资料比较
两组HSIL患者各项临床资料对比结果显示,CD3+、CD8+、IgA、IgG、IgM、LE、乳酸杆菌、BV、TV、VVC及pH对HSIL患者术后HPV转归无显著影响(P均>0.05),转阴组患者CD4+和Th/Ts水平显著高于未转阴组,且转阴组患者IL-6和TNF-α水平显著低于未转阴组,两组SIA阳性、H2O2阳性及CLE异常的患者数差异具有统计学意义(P均<0.05),见表4。
表4 转阴组和未转阴组患者临床资料比较![]() 例(%)]
例(%)]
Table 4 Comparison of clinical data between the negative conversion group and the non-negative conversion group ![]()
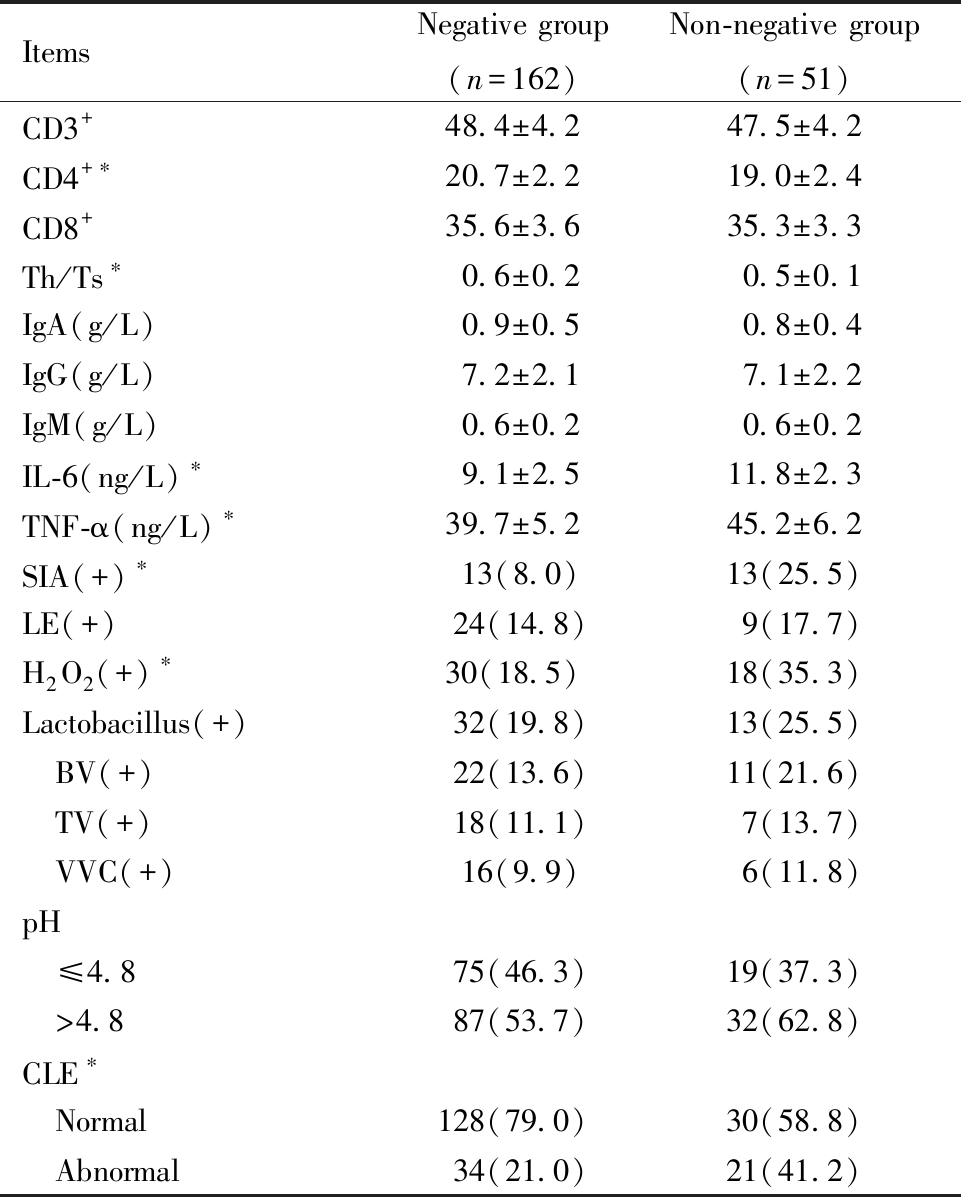
Note:Comparison betweenthe two groups,*P<0.05
ItemsNegative group(n=162)Non-negative group(n=51)CD3+48.4±4.247.5±4.2CD4+∗20.7±2.219.0±2.4CD8+35.6±3.635.3±3.3Th/Ts∗0.6±0.20.5±0.1IgA(g/L)0.9±0.50.8±0.4IgG(g/L)7.2±2.17.1±2.2IgM(g/L)0.6±0.20.6±0.2IL-6(ng/L)∗9.1±2.511.8±2.3TNF-α(ng/L)∗39.7±5.245.2±6.2SIA(+)∗13(8.0)13(25.5)LE(+)24(14.8)9(17.7)H2O2(+)∗30(18.5)18(35.3)Lactobacillus(+)32(19.8)13(25.5) BV(+)22(13.6)11(21.6) TV(+)18(11.1)7(13.7) VVC(+)16(9.9)6(11.8)pH ≤4.875(46.3)19(37.3) >4.887(53.7)32(62.8)CLE∗ Normal128(79.0)30(58.8) Abnormal34(21.0)21(41.2)
五、T淋巴细胞亚群水平与HSIL患者术后HPV转归的关系
术后12月对比两组HSIL患者T淋巴细胞亚群水平,转阴组与未转阴组患者CD3+、CD8+细胞数无显著差异(P>0.05),转阴组患者CD4+细胞数及Th/Ts水平显著高于未转阴组(P<0.05),见图2。
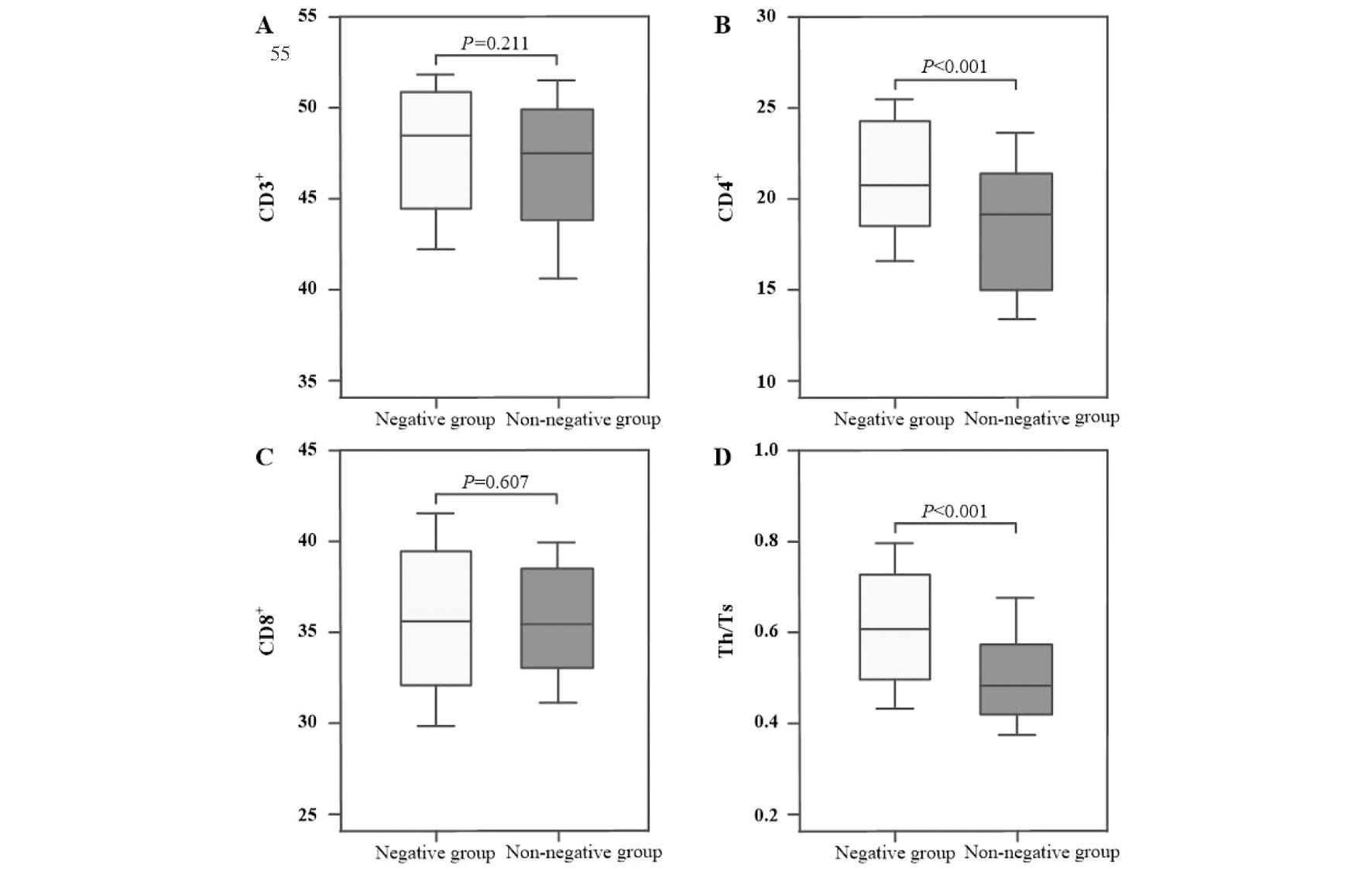
图2 HSIL患者T淋巴细胞亚群水平与术后HPV转归情况对比
Figure 2 Comparison of the level of T lymphocyte subsets and postoperative HPV outcome in HSIL patients
六、多因素分析
将HSIL患者术后HPV转归情况(转阴=0,未转阴=1)作为因变量,将单因素分析中差异具有统计学意义的因素,年龄、流产史、CD4+、Th/Ts、IL-6、TNF-α、SIA、H2O2及CLE作为自变量,赋值见表5。多因素Logistic回归分析结果显示,年龄大和TNF-α水平高是HSIL患者术后HPV未转阴的危险因素,CD4+细胞数多和Th/Ts水平高是HSIL患者术后HPV未转阴的保护因素(P<0.05),见表6。
表5 各因素赋值情况
Table 5 Assignment of each factor
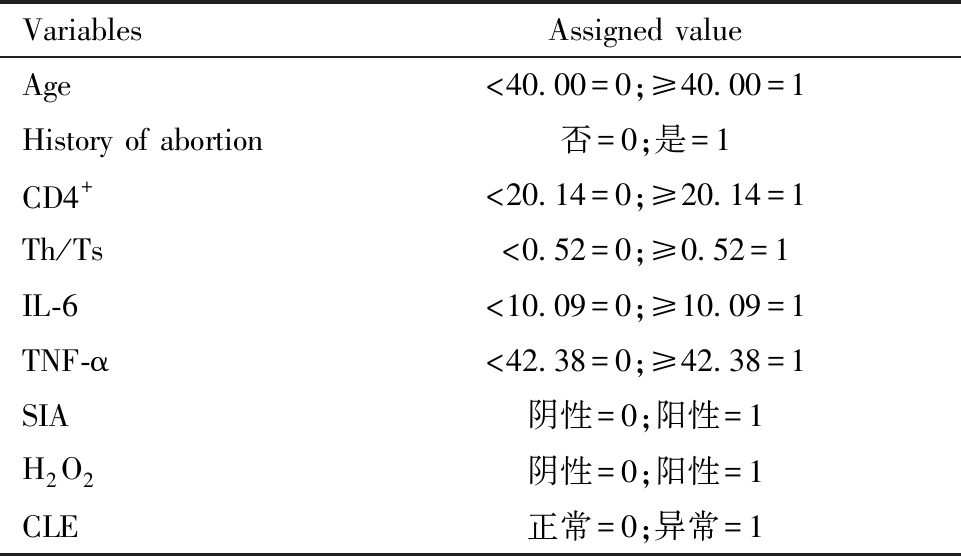
VariablesAssigned valueAge<40.00=0;≥40.00=1History of abortion否=0;是=1CD4+<20.14=0;≥20.14=1Th/Ts<0.52=0;≥0.52=1IL-6<10.09=0;≥10.09=1TNF-α<42.38=0;≥42.38=1SIA阴性=0;阳性=1H2O2阴性=0;阳性=1CLE正常=0;异常=1
表6 HSIL患者术后HPV转归多因素分析
Table 6 Multivariate analysis of postoperative HPV outcomes in HSIL patients
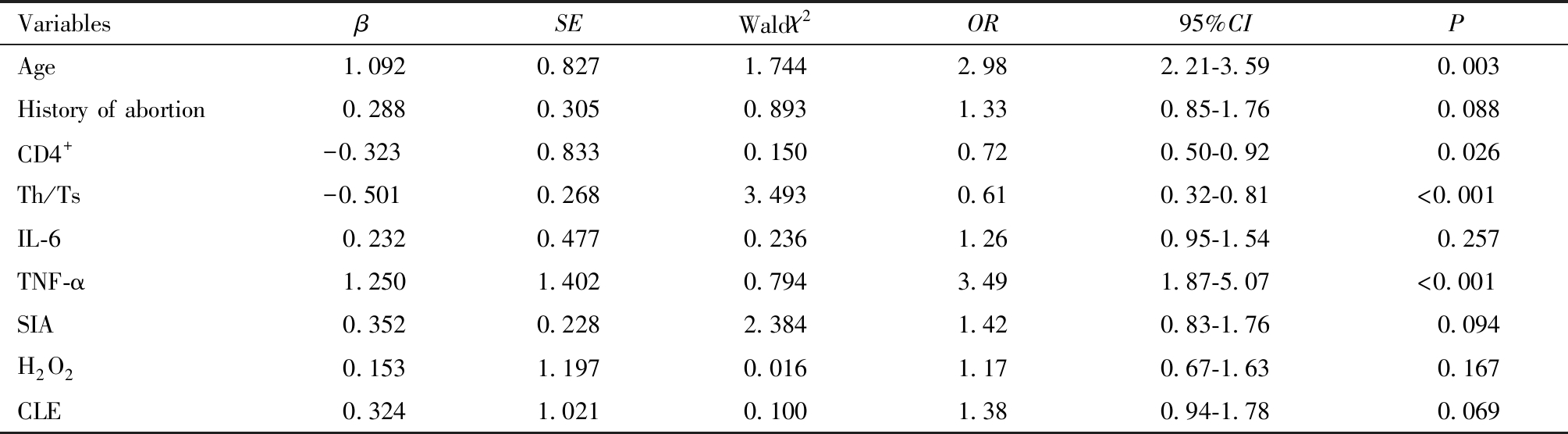
VariablesβSEWaldχ2OR95%CIPAge 1.0920.8271.7442.982.21-3.59 0.003History of abortion 0.2880.3050.8931.330.85-1.76 0.088CD4+-0.3230.8330.1500.720.50-0.92 0.026Th/Ts-0.5010.2683.4930.610.32-0.81<0.001IL-6 0.2320.4770.2361.260.95-1.54 0.257TNF-α 1.2501.4020.7943.491.87-5.07<0.001SIA 0.3520.2282.3841.420.83-1.76 0.094H2O2 0.1531.1970.0161.170.67-1.63 0.167CLE 0.3241.0210.1001.380.94-1.78 0.069
七、HSIL患者术后HPV转归预测效能比较
利用年龄、CD4+、Th/Ts及TNF-α水平单独检验预测HSIL患者术后HPV转归情况的ROC曲线AUC分别为0.714、0.676、0.778、0.730,见表7和图3。年龄+CD4++Th/Ts+TNF-α联合检测HSIL患者术后HPV转归情况AUC为0.881,高于任何单一因素预测模型,且约登指数和准确性也高于其他模型,见表7和图3。
表7 HSIL患者术后HPV转归预测效能对比
Table 7 Comparison of predictive efficacy of postoperative HPV outcomes in HSIL patients
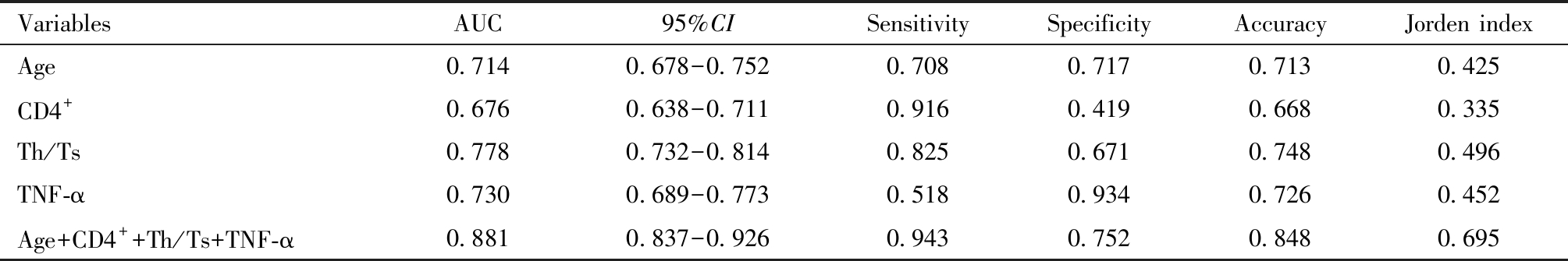
VariablesAUC95%CISensitivitySpecificityAccuracyJorden indexAge0.7140.678-0.7520.7080.7170.7130.425CD4+0.6760.638-0.7110.9160.4190.6680.335Th/Ts0.7780.732-0.8140.8250.6710.7480.496TNF-α0.7300.689-0.7730.5180.9340.7260.452Age+CD4++Th/Ts+TNF-α0.8810.837-0.9260.9430.7520.8480.695
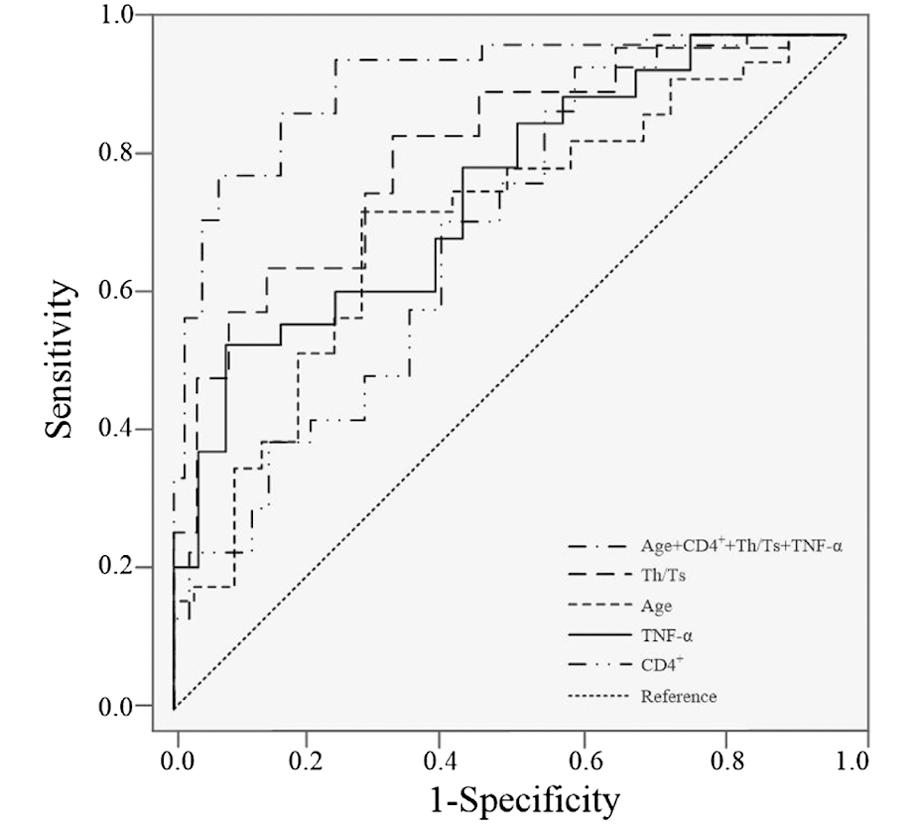
图3 年龄、CD4+、Th/Ts及TNF-α水平预测HSIL患者术后HPV转归的ROC曲线
Figure 3 ROC curve of age, CD4+, Th/Ts and TNF-α levels predicting HPV outcome in patients with HSIL
八、危险因素与HPV转归关联性分析
将年龄、CD4+、Th/Ts及TNF-α水平逐层划分(Q1-Q5),建立Logistic模型逐步排除存在共线性的混杂因素,最终校正饮酒、主动或被动吸烟、孕次、产次、病程、流产史、IL-6、SIA、H2O2、CLE,以消除混杂因素对HPV转归的影响,结果见表8。在未经调整的Logistic模型(未校正模型)中,年龄、CD4+、Th/Ts及TNF-α水平与ICP患者妊娠结局显著相关(P<0.001),调整后(模型5),年龄(OR=1.85,95% CI:1.57~2.13)、CD4+(OR=1.69,95% CI:1.41~1.95)、Th/Ts(OR=2.18,95% CI:1.94~2.43)及TNF-α(OR=1.86,95% CI:1.60~2.13)仍为HSIL患者术后HPV转归的独立影响因素。随着年龄、TNF-α的升高(Q2-Q5),CD4+、Th/Ts水平的降低(Q2-Q5),其关联效应值也相应升高,趋势性检验差异均有统计学意义(P趋势<0.05)。
表8 不同年龄、CD4+、Th/Ts及TNF-α对HSIL患者术后HPV转归的关联效应分析
Table 8 Analysis about the association effect of different levels of age, CD4+, Th/Ts and TNF-α on postoperative HPV outcome in patients with HSIL
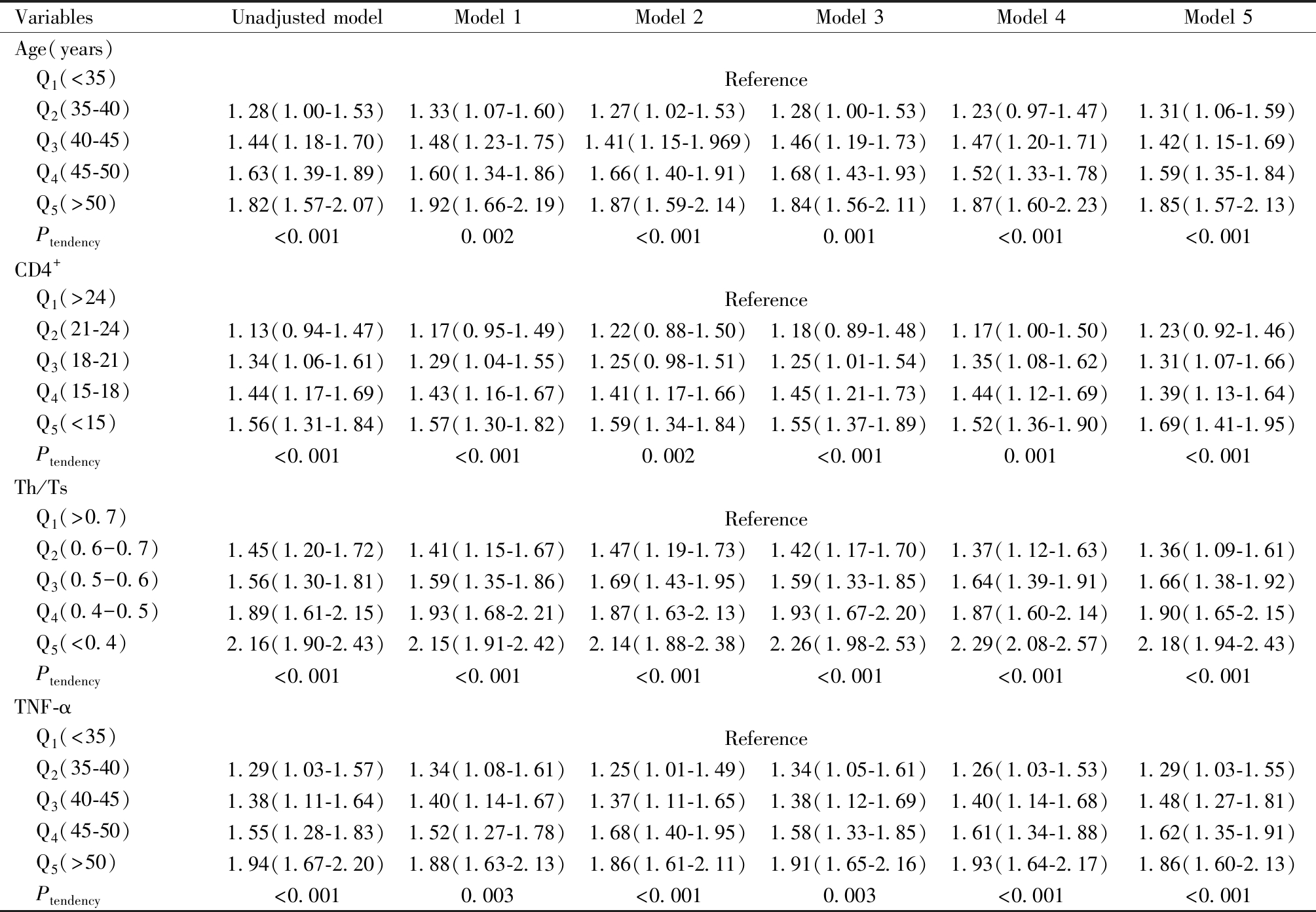
Note:Model 1=Adjusted for history of abortion、levels of IL-6、SIA、H2O2 and CLE,Model 2=Model 1+Age,Model 3=Model 2+TNF-α,Model 4=Model 3+CD4+,Model 5=Model 4+Th/Ts
VariablesUnadjusted modelModel 1Model 2Model 3Model 4Model 5Age(years) Q1(<35)Reference Q2(35-40)1.28(1.00-1.53)1.33(1.07-1.60)1.27(1.02-1.53)1.28(1.00-1.53)1.23(0.97-1.47)1.31(1.06-1.59) Q3(40-45)1.44(1.18-1.70)1.48(1.23-1.75)1.41(1.15-1.969)1.46(1.19-1.73)1.47(1.20-1.71)1.42(1.15-1.69) Q4(45-50)1.63(1.39-1.89)1.60(1.34-1.86)1.66(1.40-1.91)1.68(1.43-1.93)1.52(1.33-1.78)1.59(1.35-1.84) Q5(>50)1.82(1.57-2.07)1.92(1.66-2.19)1.87(1.59-2.14)1.84(1.56-2.11)1.87(1.60-2.23)1.85(1.57-2.13) Ptendency<0.0010.002<0.0010.001<0.001<0.001CD4+ Q1(>24)Reference Q2(21-24)1.13(0.94-1.47)1.17(0.95-1.49)1.22(0.88-1.50)1.18(0.89-1.48)1.17(1.00-1.50)1.23(0.92-1.46) Q3(18-21)1.34(1.06-1.61)1.29(1.04-1.55)1.25(0.98-1.51)1.25(1.01-1.54)1.35(1.08-1.62)1.31(1.07-1.66) Q4(15-18)1.44(1.17-1.69)1.43(1.16-1.67)1.41(1.17-1.66)1.45(1.21-1.73)1.44(1.12-1.69)1.39(1.13-1.64) Q5(<15)1.56(1.31-1.84)1.57(1.30-1.82)1.59(1.34-1.84)1.55(1.37-1.89)1.52(1.36-1.90)1.69(1.41-1.95) Ptendency<0.001<0.0010.002<0.0010.001<0.001Th/Ts Q1(>0.7)Reference Q2(0.6-0.7)1.45(1.20-1.72)1.41(1.15-1.67)1.47(1.19-1.73)1.42(1.17-1.70)1.37(1.12-1.63)1.36(1.09-1.61) Q3(0.5-0.6)1.56(1.30-1.81)1.59(1.35-1.86)1.69(1.43-1.95)1.59(1.33-1.85)1.64(1.39-1.91)1.66(1.38-1.92) Q4(0.4-0.5)1.89(1.61-2.15)1.93(1.68-2.21)1.87(1.63-2.13)1.93(1.67-2.20)1.87(1.60-2.14)1.90(1.65-2.15) Q5(<0.4)2.16(1.90-2.43)2.15(1.91-2.42)2.14(1.88-2.38)2.26(1.98-2.53)2.29(2.08-2.57)2.18(1.94-2.43) Ptendency<0.001<0.001<0.001<0.001<0.001<0.001TNF-α Q1(<35)Reference Q2(35-40)1.29(1.03-1.57)1.34(1.08-1.61)1.25(1.01-1.49)1.34(1.05-1.61)1.26(1.03-1.53)1.29(1.03-1.55) Q3(40-45)1.38(1.11-1.64)1.40(1.14-1.67)1.37(1.11-1.65)1.38(1.12-1.69)1.40(1.14-1.68)1.48(1.27-1.81) Q4(45-50)1.55(1.28-1.83)1.52(1.27-1.78)1.68(1.40-1.95)1.58(1.33-1.85)1.61(1.34-1.88)1.62(1.35-1.91) Q5(>50)1.94(1.67-2.20)1.88(1.63-2.13)1.86(1.61-2.11)1.91(1.65-2.16)1.93(1.64-2.17)1.86(1.60-2.13) Ptendency<0.0010.003<0.0010.003<0.001<0.001
讨 论
CC是原发于子宫颈部位的恶性肿瘤,为女性生殖道最常见的恶性肿瘤,其发病早期缺乏典型临床表现,导致部分患者确诊时就已经是中晚期,从而错失最佳治疗时机,对生命安全造成威胁[11]。HSIL是宫颈癌前病变的典型表现,目前已明确的主要致病原因为HPV的持续感染[12]。LEEP刀宫颈锥切术能有效切除病变组织和清除HPV,降低CIN进一步癌变风险,但有些潜藏于皮肤深处的HPV无法完全消除,并且LEEP切口由于热损伤导致病变组织残留,可能会导致HPV持续感染甚至CIN复发[13-14]。HPV不仅存在于宫颈病变组织中,还能在阴道上皮组织等部位检测出,LEEP刀宫颈锥切术无法清除宫颈以外的HPV,只能依靠患者自身免疫力清除残留HPV,绝大多数HSIL患者术后HPV感染逐渐转阴,但少部分患者术后病变组织残留、免疫功能紊乱,导致HPV持续感染和病情复发[15]。因此,HSIL患者机体免疫应答与术后HPV转归关系的研究尤为重要,对临床治疗HSIL和清除HPV具有指导意义。
T细胞是细胞免疫功能中最重要的细胞群,而免疫球蛋白(immunoglobulin,Ig)是人体体液免疫功能中最重要的蛋白家族,当人体细胞和体液免疫应答产生变化时,T细胞和Ig水平也会随之变化[16]。本研究中HSIL患者术后12月复诊时CD3+、CD4+、Th/Ts、IgA、IgG、IgM水平显著升高,CD8+细胞数则显著降低,同时还发现CD4+细胞数及Th/Ts水平为HSIL患者术后HPV转归的独立影响因素。李世蓉[17]等研究也认为,CD4+百分比及CD4+/CD8+比值的消长对不同宫颈病变HPV感染的转归起着重要作用。HPV持续感染会对细胞免疫和体液免疫造成不同程度损伤,大量抗体和抗原被消耗,导致患者自身免疫无法完全清除HPV,进一步降低机体免疫力[18]。有研究表示CIN术后恢复情况与患者年龄和TNF-α水平有关[19-22],本研究也有类似发现,年龄大和TNF-α水平高均为影响HSIL患者术后HPV转归的危险因素,可能是由于患者雌激素的分泌随年龄增加而逐渐减少,阴道黏膜萎缩、变薄,导致阴道抵抗力降低,引起残留HPV持续感染[23]。而TNF-α生物学功能包括抗肿瘤、免疫调节、介导炎症等,TNF-α的过量产生,暗示了一些病理条件下所起的重要作用,包括疾病恶化、免疫紊乱等,影响了HSIL患者HPV的转归[24]。本研究利用年龄、CD4+、Th/Ts及TNF-α水平预测HSIL患者术后HPV转归情况发现,年龄+CD4++Th/Ts+TNF-α联合检测HSIL患者术后HPV转归情况AUC为0.881,高于任何单一因素预测模型,且约登指数和准确性分别为0.695、0.848,均高于其他预测模型。将年龄、CD4+、Th/Ts及TNF-α水平逐层划分分析其与HPV转归关联性,消除混杂因素后分析结果显示随着年龄、TNF-α的升高,和CD4+、Th/Ts水平的降低,其关联效应值也相应升高。
本研究尚存在一定的局限性,纳入的样本数据来自同一医疗中心,结果难免存在一定偏倚;研究缺少术后12月后相关数据分析,分析结果的深度和广度需进一步提升,可将远期预后相关数据分析作为未来研究重点。
综上所述,与术前相比,术后12月HSIL患者细胞免疫应答和体液免疫应答得到显著改善;年龄大和TNF-α水平高是HSIL患者术后HPV未转阴的危险因素,CD4+细胞数多及Th/Ts水平高是HSIL患者术后HPV未转阴的保护因素;年龄+CD4++Th/Ts+TNF-α联合检测HSIL患者术后HPV转归情况,其预测性能较好;消除混杂因素后,随着年龄、TNF-α的升高,CD4+细胞数、Th/Ts水平的降低,其与HPV转归的关联效应值也相应升高。
1 Zhang J,Cheng K,Wang Z.Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China:a meta-analysis.Arch Gynecol Obstet,2020,302:1329-1337.
2 中华医学会妇科肿瘤学分会,中国优生科学协会阴道镜和宫颈病理学分会,马丁,等.人乳头瘤病毒疫苗临床应用中国专家共识.协和医学杂志,2021,12:189-201.
3 董蔼玲,宋晓婕,孔繁丽,等.宫颈上皮内瘤变患者术后HR-HPV持续感染影响因素.中华医院感染学杂志,2021,31:3191-3195.
4 McBride AA.Human papillomaviruses:diversity,infection and host interactions.Nat Rev Microbiol,2022,20:95-108.
5 Monti M,D′Aniello D,Scopelliti A,et al.Relationship between cervical excisional treatment for cervical intraepithelial neoplasia and obstetrical outcome.Minerva Obstet Gynecol,2021,73:233-246.
6 林一禾,朱淑嫔,陈游沓.高危型HPV持续感染者宫颈局部调节性T淋巴细胞表达.中国计划生育学杂志,2021,29:2208-2210,2214.
7 王伊洁,杨玮丽,李慰,等.宫颈高危型人乳头瘤病毒感染患者阴道微生态和外周血T淋巴细胞亚群分析.中国妇幼保健,2021,36:3342-3346.
8 Abdulaziz A,You X,Liu L,et al.Management of high-grade squamous intraepithelial lesion patients with positive margin after LEEP conization:A retrospective study.Medicine(Baltimore),2021,100:e26030.
9 谢幸.妇产科学.第9版,北京:人民卫生出版社,2018:402-403.
10 中华医学会妇产科学分会感染性疾病协作组.阴道微生态评价的临床应用专家共识.中华妇产科杂志,2016,51:721-723.
11 中国抗癌协会妇科肿瘤专业委员会.子宫颈癌诊断与治疗指南(2021年版).中国癌症杂志,2021,31:474-489.
12 Reich O,Regauer S,Kashofer K.Possibly carcinogenic HPV subtypes are a cause of HSIL and negative clinical HPV tests - A European prospective single center study.Gynecol Oncol,2020,158:112-116.
13 Zang L,Huang J,Zhu J,et al.Risk factors associated with the persistence of human papillomavirus after cervical excision in patients with high-grade squamous intra-epithelial neoplasia.Eur J Obstet Gynecol Reprod Biol,2021,266:175-181.
14 Kim M,Choi MC,Lee C,et al.Long-term outcomes of photodynamic therapy for a positive resection margin after conization for cervical intraepithelial neoplasia grade 3.Photodiagnosis Photodyn Ther,2022,37:102639.
15 Ouh YT,Cho HW,Kim SM,et al.Risk factors for type-specific persistence of high-risk human papillomavirus and residual/recurrent cervical intraepithelial neoplasia after surgical treatment.Obstet Gynecol Sci,2020,63:631-642.
16 Mitra A,MacIntyre DA,Paraskevaidi M,et al.The vaginal microbiota and innate immunity after local excisional treatment for cervical intraepithelial neoplasia.Genome Med,2021,13:176.
17 李世蓉,张三元,刘冬青,等.IgG、INF-γ及CD4~+/CD8~+T细胞对不同宫颈病变高危型HPV感染转归的影响.中国妇幼保健,2015,30:5125-5126.
18 McBride AA.Human papillomaviruses:diversity,infection and host interactions.Nat Rev Microbiol,2022,20:95-108.
19 张菲菲,高广云,张海燕,等.宫颈上皮内瘤变患者宫颈环形电切术后HPV持续感染的高危因素分析及其对阴道微生态和免疫调节功能的影响.现代生物医学进展,2022,22:2854-2859.
20 秦燕萍,杜鹏,杨丽芳.LEEP联合重组人干扰素α-2 b凝胶治疗宫颈上皮内瘤变的疗效及对患者免疫功能的影响.中国性科学,2020,29:29-32.
21 申鑫鑫,豆贝贝,滑天,等.高危型HPV16感染阴道灌洗液中TNF-α及IL-12和IL-10表达与宫颈上皮内瘤变级别的相关性.中华医院感染学杂志,2021,31:1419-1422.
22 Vänskä S,Luostarinen T,Lagheden C,et al.Differing age-specific cervical cancer incidence between different types of human papillomavirus:implications for predicting the impact of elimination programs.Am J Epidemiol,2021,190:506-514.
23 Jayshree RS.The immune microenvironment in human papillomavirus-induced cervical lesions-evidence for estrogen as an immunomodulator.Front Cell Infect Microbiol,2021,11:649815.
24 Behboodi N,Farazestanian M,Rastgar-Moghadam A,et al.Association of a variant in the tumor necrosis factor alpha gene with risk of cervical cancer.Mol Biol Rep,2021,48:1433-1437.