子宫内膜癌(endometrial carcinoma,EC)是女性生殖系统常见的妇科恶性肿瘤,2020年全球诊断出41.7万例子宫内膜癌,在过去30年,发病率上升了132%,严重影响女性生殖健康[1]。80% 的 EC 患者在早期被诊断并预后良好,5 年生存率约为80%~85%[2]。EC的标准治疗方法是全子宫切除术和双侧输卵管卵巢切除术[3]。
临床上主要通过患者的临床表现、妇科检查及辅助检查进行EC的诊断,其确诊依赖于子宫内膜活检[4-5]。诊断性刮宫术属于盲性操作,存在漏诊的可能性,并伴有一定的癌症扩散风险。相比之下,宫腔镜检查在准确取材方面具有显著优势,但对于子宫肌层浸润深度及肿瘤大小的判断能力仍有所不足。目前认为,非子宫内膜样癌、宫颈间质浸润、肿瘤直径、深肌层浸润、组织学分级、脉管浸润、宫颈间隙浸润和远处转移等病理特征均为影响EC预后的影响因素[6-8]。近年来,研究者越来越重视血清肿瘤标志物在EC的临床应用价值,有研究发现血清CA125、HE4及CEA在EC的早期诊断与宫外转移监测中具有潜在价值[9-10]。但临床关于其与子宫内膜癌病理特征之间的关系研究较少。此外,肿瘤的第七大显著特征为炎症反应,这一反应在肿瘤的发生与发展过程中均扮演着至关重要的角色,肿瘤引起的炎症反应可使血液中的某些成分发生变化[11]。目前尚无临床批准的用于EC诊断和预后的外周血指标。本文通过分析鳞状细胞癌抗原(squamous cell carcinoma antigen,SCC)、糖类抗原 125(carbohydrate antigen 125,CA125)、糖类抗原 199(carbohydrate antigen 199,CA199)、E4(human epididymal protein E4,HE4)、癌胚抗原(carcinoembryonic antigen,CEA)及甲胎蛋白(alpha fetoprotein,AFP)6 种肿瘤标志物及外周血中性粒细胞与淋巴细胞计数比(neutrophil to lymphocyte ratio,NLR)、单核与淋巴细胞计数比(monocyte to lymphocyte ratio,MLR)和血小板与淋巴细胞计数比(platelet to lymphocyte ratio,PLR)与肿瘤肌层浸润深度及肿瘤大小间的内在联系及其诊断价值,为临床工作者临床决策提供一些参考价值,为评估EC预后提供新思路。
对象与方法
一、对象
选取 2018 年1月—2023年7月山西医科大学第一临床医学院收治的229例子宫内膜病变患者,其中144例子宫内膜癌患者为子宫内膜癌组,85例子宫内膜非典型增生(atypical hyperplasia of endometrium,AHE)患者为子宫内膜非典型增生组。144例子宫内膜癌患者均行全子宫+左右盆腔淋巴结清洁术。两组患者的纳入标准:(1)因子宫内膜病变就诊,病例资料完整;(2)经病理学检查确诊为EC或AHE;(3)术前未接受过新辅助治疗、激素及免疫抑制剂治疗。排除标准:(1)近期伴有急性或慢性感染患者;(2)合并心、肝、肾等重要脏器疾病;(3)合并宫体、宫颈、输卵管等其他部位肿瘤。144 例子宫内膜癌患者的年龄为 49.2~59.0 岁,中位年龄为54.0岁;病理分型:子宫内膜样腺癌133例,透明细胞癌6例,浆液性乳头状癌4例,癌肉瘤1例;采用国际妇产科联盟(FIGO)分期标准进行临床分期:Ⅰ期117例,Ⅱ期8例,Ⅲ期18例,Ⅳ期1例。85例子宫内膜非典型增生患者的年龄为39.5~51.5岁,中位年龄为47.0岁。
二、方法
1. 一般临床资料:收集两组患者发病年龄、初潮年龄、是否绝经、是否异常子宫出血及流液、病程持续时间是否≥1年、体质指数(body mass index,BMI)、是否合并高血压、糖尿病。
2.血液指标:所有研究对象入院后空腹采集5 mL静脉血,以3 000 r/min速度离心10 min,之后以酶联免疫吸附法测定血清CA125、CA199、CEA、AFP、SCC、HE4水平,试剂盒购自上海江莱生物科技有限公司,所有检测均严格按照试剂盒要求开展。应用ACLTOP750全自动血凝仪检测:D-二聚体(D-dimer,D-D)、纤维蛋白原(fibrinogen,FIB)、血小板计数(platelet count,PLT)、中性粒细胞数(neutrophil count,N)、淋巴细胞数(lymphocyte count,L)、单核细胞数(monocyte count,M)、白细胞计数(white blood cell count,WBC)。正常参考范围:CA125≤35 U/mL,CA199≤37 U/mL,CEA≤5 μg/L,AFP≤20 IU/mL,SCC≤1.5 ng/mL,HE4≤140 pmol/L。计算NLR(NLR=中性粒细胞计数/淋巴细胞计数)、PLR(PLR=血小板计数/淋巴细胞计数)和MLR(MLR=单核细胞计数/淋巴细胞计数)。
3.术后病理资料:收集患者病理类型、肿瘤大小、组织学分级、肌层浸润深度、是否淋巴结转移、淋巴血管间隙浸润、宫颈间质浸润、附件受累等。
4. 统计学处理:采用 SPSS 27.0 软件对数据进行统计分析。计量资料均经 Shapiro -wilk 正态性检验,符合正态分布的计量资料以![]() 表示,若满足方差齐性,组间比较采用独立样本t检验;非正态分布的计量资料以中位数(四分位数)[M(P25,P75)]表示,采用Mann-Whitney U检验进行组间对比;计数资料以例数及率(%)表示,运用χ2检验或 Fisher确切概率法进行分析;发生子宫内膜癌深肌层浸润及肿瘤直径>2 cm的影响因素分析采用Logistic 回归。绘制受试者工作特征曲线(receiver operator characteristic,ROC)检验子宫内膜癌患者血清CA125及CEA水平对肌层深浸润的诊断效能和血清CA125及HE4水平对肿瘤直径>2 cm的诊断效能,以曲线下面积(area under cure,AUC)评价,P<0.05为差异有统计学意义。
表示,若满足方差齐性,组间比较采用独立样本t检验;非正态分布的计量资料以中位数(四分位数)[M(P25,P75)]表示,采用Mann-Whitney U检验进行组间对比;计数资料以例数及率(%)表示,运用χ2检验或 Fisher确切概率法进行分析;发生子宫内膜癌深肌层浸润及肿瘤直径>2 cm的影响因素分析采用Logistic 回归。绘制受试者工作特征曲线(receiver operator characteristic,ROC)检验子宫内膜癌患者血清CA125及CEA水平对肌层深浸润的诊断效能和血清CA125及HE4水平对肿瘤直径>2 cm的诊断效能,以曲线下面积(area under cure,AUC)评价,P<0.05为差异有统计学意义。
结 果
一、两组患者临床病理特征
子宫内膜癌组与子宫内膜非典型增生组的两组研究对象初潮年龄、有异常阴道出血病史,病史时间≥1年、合并高血压史等比较,差异均无统计学意义(P>0.05)。子宫内膜癌组与子宫内膜非典型增生组术前血清SCC、CA125、AFP、NLR、PLR、MLR水平比较,差异无统计学意义(P>0.05);子宫内膜癌组中术前血清CA199、CEA、HE4、D-D、FIB水平与子宫内膜非典型增生组,差异均有统计学意义(P<0.05),见表1。
表1 AHE组和EC组患者一般资料比较 [M(P25,P75)]
Table 1 Comparison of general data of patients in the AHE group and the EC group [M(P25, P75)]
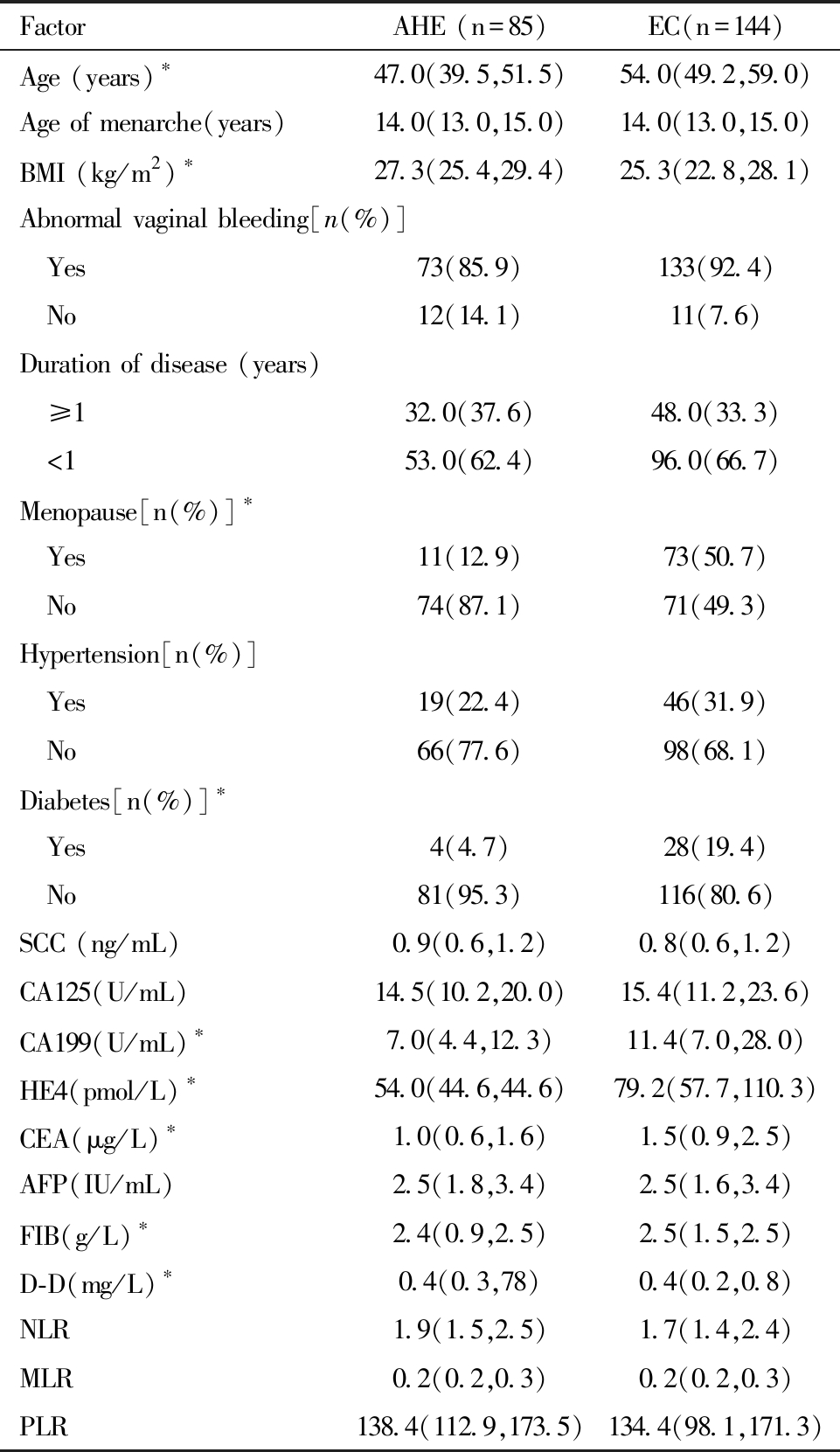
Comparison between the two groups,*P<0.05
FactorAHE (n=85)EC(n=144)Age (years)∗47.0(39.5,51.5)54.0(49.2,59.0)Age of menarche(years)14.0(13.0,15.0)14.0(13.0,15.0)BMI (kg/m2)∗27.3(25.4,29.4)25.3(22.8,28.1)Abnormal vaginal bleeding[n(%)] Yes73(85.9)133(92.4) No12(14.1)11(7.6)Duration of disease (years) ≥132.0(37.6)48.0(33.3) <153.0(62.4)96.0(66.7)Menopause[n(%)]∗ Yes11(12.9)73(50.7) No74(87.1)71(49.3)Hypertension[n(%)] Yes19(22.4)46(31.9) No66(77.6)98(68.1)Diabetes[n(%)]∗ Yes4(4.7)28(19.4) No81(95.3)116(80.6)SCC (ng/mL)0.9(0.6,1.2)0.8(0.6,1.2)CA125(U/mL)14.5(10.2,20.0)15.4(11.2,23.6)CA199(U/mL) ∗7.0(4.4,12.3)11.4(7.0,28.0)HE4(pmol/L) ∗54.0(44.6,44.6)79.2(57.7,110.3)CEA(μg/L) ∗1.0(0.6,1.6)1.5(0.9,2.5)AFP(IU/mL)2.5(1.8,3.4)2.5(1.6,3.4)FIB(g/L) ∗2.4(0.9,2.5)2.5(1.5,2.5)D-D(mg/L) ∗0.4(0.3,78)0.4(0.2,0.8)NLR1.9(1.5,2.5)1.7(1.4,2.4)MLR0.2(0.2,0.3)0.2(0.2,0.3)PLR138.4(112.9,173.5)134.4(98.1,171.3)
二、术前肿瘤标志物及炎症指标与子宫内膜癌病理特征关系分析
肿瘤直径>2 cm患者的术前CA125、HE4、NLR、PLR与MLR均显著高于肿瘤直径≤2 cm患者,且差异有统计学意义(P<0.05)。肌层浸润深度≥1/2 患者的术前 CA125、CA199、HE4、CEA、MLR值高于浸润深度<1/2 患者,差异有统计学意义(P<0.05)。见表2及表3。
表2 血清肿瘤标志物与子宫内膜癌各临床病理参数的关系[M(P25,P75)]
Table 2 Relationship between serum tumor markers and clinicopathological parameters of endometrial cancer [M (P25, P75)]
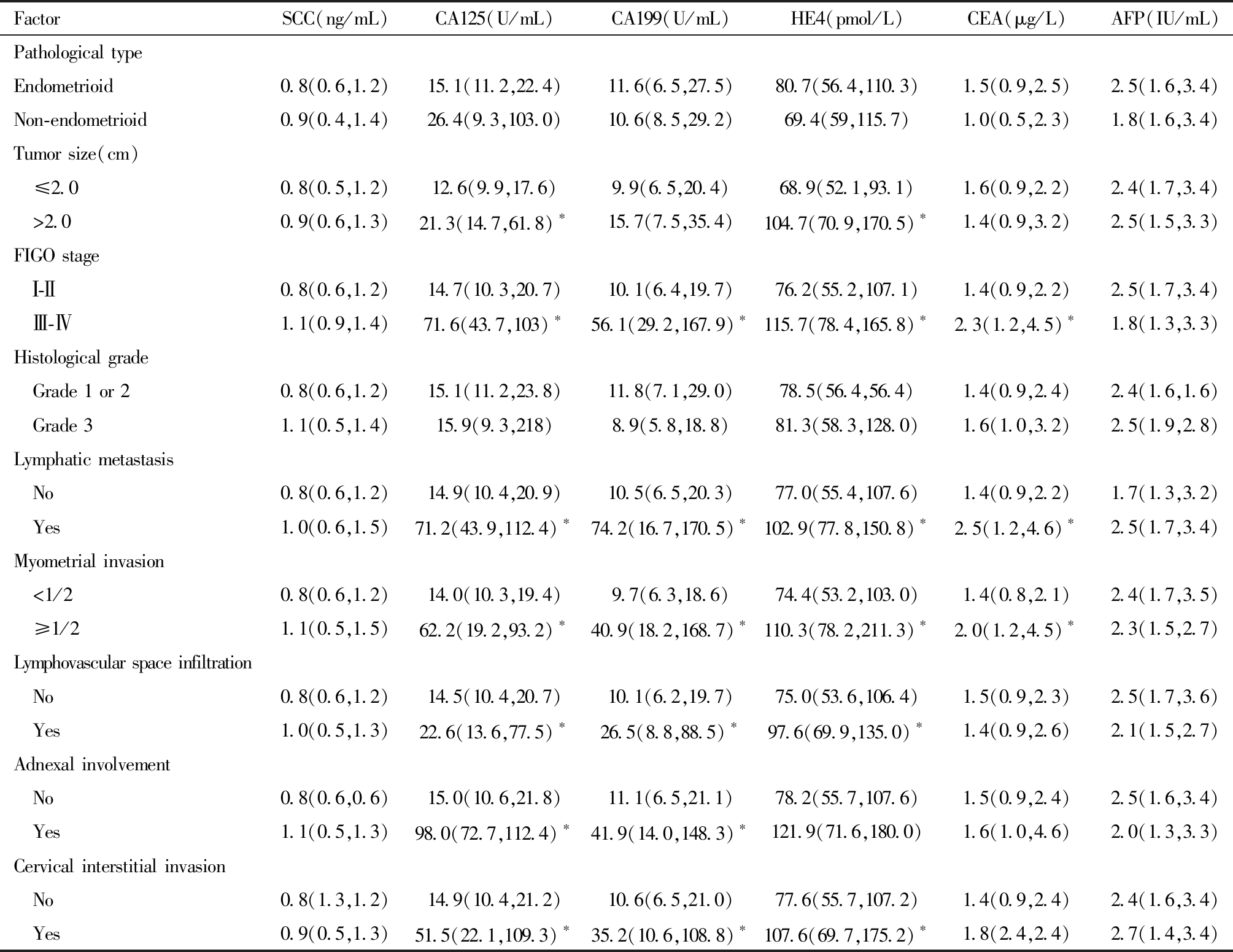
Comparison between the two groups, *P<0.05
FactorSCC(ng/mL)CA125(U/mL)CA199(U/mL)HE4(pmol/L)CEA(μg/L)AFP(IU/mL)Pathological typeEndometrioid0.8(0.6,1.2)15.1(11.2,22.4)11.6(6.5,27.5)80.7(56.4,110.3)1.5(0.9,2.5)2.5(1.6,3.4)Non-endometrioid0.9(0.4,1.4)26.4(9.3,103.0)10.6(8.5,29.2)69.4(59,115.7)1.0(0.5,2.3)1.8(1.6,3.4)Tumor size(cm) ≤2.00.8(0.5,1.2)12.6(9.9,17.6)9.9(6.5,20.4)68.9(52.1,93.1)1.6(0.9,2.2)2.4(1.7,3.4) >2.00.9(0.6,1.3)21.3(14.7,61.8)∗15.7(7.5,35.4)104.7(70.9,170.5)∗1.4(0.9,3.2)2.5(1.5,3.3)FIGO stage Ⅰ-Ⅱ0.8(0.6,1.2)14.7(10.3,20.7)10.1(6.4,19.7)76.2(55.2,107.1)1.4(0.9,2.2)2.5(1.7,3.4) Ⅲ-Ⅳ1.1(0.9,1.4)71.6(43.7,103)∗56.1(29.2,167.9)∗115.7(78.4,165.8)∗2.3(1.2,4.5)∗1.8(1.3,3.3)Histological grade Grade 1 or 20.8(0.6,1.2)15.1(11.2,23.8)11.8(7.1,29.0)78.5(56.4,56.4)1.4(0.9,2.4)2.4(1.6,1.6) Grade 31.1(0.5,1.4)15.9(9.3,218)8.9(5.8,18.8)81.3(58.3,128.0)1.6(1.0,3.2)2.5(1.9,2.8)Lymphatic metastasis No0.8(0.6,1.2)14.9(10.4,20.9)10.5(6.5,20.3)77.0(55.4,107.6)1.4(0.9,2.2)1.7(1.3,3.2) Yes1.0(0.6,1.5)71.2(43.9,112.4)∗74.2(16.7,170.5)∗102.9(77.8,150.8)∗2.5(1.2,4.6)∗2.5(1.7,3.4)Myometrial invasion <1/20.8(0.6,1.2)14.0(10.3,19.4)9.7(6.3,18.6)74.4(53.2,103.0)1.4(0.8,2.1)2.4(1.7,3.5) ≥1/21.1(0.5,1.5)62.2(19.2,93.2)∗40.9(18.2,168.7)∗110.3(78.2,211.3)∗2.0(1.2,4.5)∗2.3(1.5,2.7)Lymphovascular space infiltration No0.8(0.6,1.2)14.5(10.4,20.7)10.1(6.2,19.7)75.0(53.6,106.4)1.5(0.9,2.3)2.5(1.7,3.6) Yes1.0(0.5,1.3)22.6(13.6,77.5)∗26.5(8.8,88.5)∗97.6(69.9,135.0)∗1.4(0.9,2.6)2.1(1.5,2.7)Adnexal involvement No0.8(0.6,0.6)15.0(10.6,21.8)11.1(6.5,21.1)78.2(55.7,107.6)1.5(0.9,2.4)2.5(1.6,3.4) Yes1.1(0.5,1.3)98.0(72.7,112.4)∗41.9(14.0,148.3)∗121.9(71.6,180.0)1.6(1.0,4.6)2.0(1.3,3.3)Cervical interstitial invasion No0.8(1.3,1.2)14.9(10.4,21.2)10.6(6.5,21.0)77.6(55.7,107.2)1.4(0.9,2.4)2.4(1.6,3.4) Yes0.9(0.5,1.3)51.5(22.1,109.3)∗35.2(10.6,108.8)∗107.6(69.7,175.2)∗1.8(2.4,2.4)2.7(1.4,3.4)
表3 术前外周血指标与子宫内膜癌各临床病理参数的关系[M(P25, P75)]
Table 3 Relationship between preoperative peripheral blood indicators and various clinicopathological parameters of endometrial cancer [M (P25, P75)]

FactorNLRMLRPLRD-D(mg/L)FIB(g/L)Pathological type Endometrioid1.7(1.3,1.3)0.2(0.2,0.3)132.6(99.4,172.6)0.4(0.2,0.6)2.5(1.5,2.5) Non-endometrioid1.8(1.5,2.9)0.2(0.1,0.3)140.5(94.6,159.8)1.0(0.4,7.9)∗2.7(1.6,9.5)Tumor size(cm) ≤2.01.6(1.3,2.2)0.2(0.2,0.2)126.1(88.8,161.1)0.3(0.2,0.9)2.5(1.3,2.5) >2.01.9(1.5,2.7)∗0.2(0.2,0.3)∗149.7±50.7∗0.4(0.3,0.8)2.5(1.8,2.5)FIGO stage Ⅰ-Ⅱ1.7(1.3,2.3)0.2(0.2,0.2)131.7(95.9,165.8)0.4(0.2,0.8)2.5(1.5,2.5) Ⅲ-Ⅳ2(1.6,2.8)∗0.3(0.2,0.3)153.3(118.2,194.3)0.4(0.3,2.2)∗2.5(1.8,5.4)
表3(续)
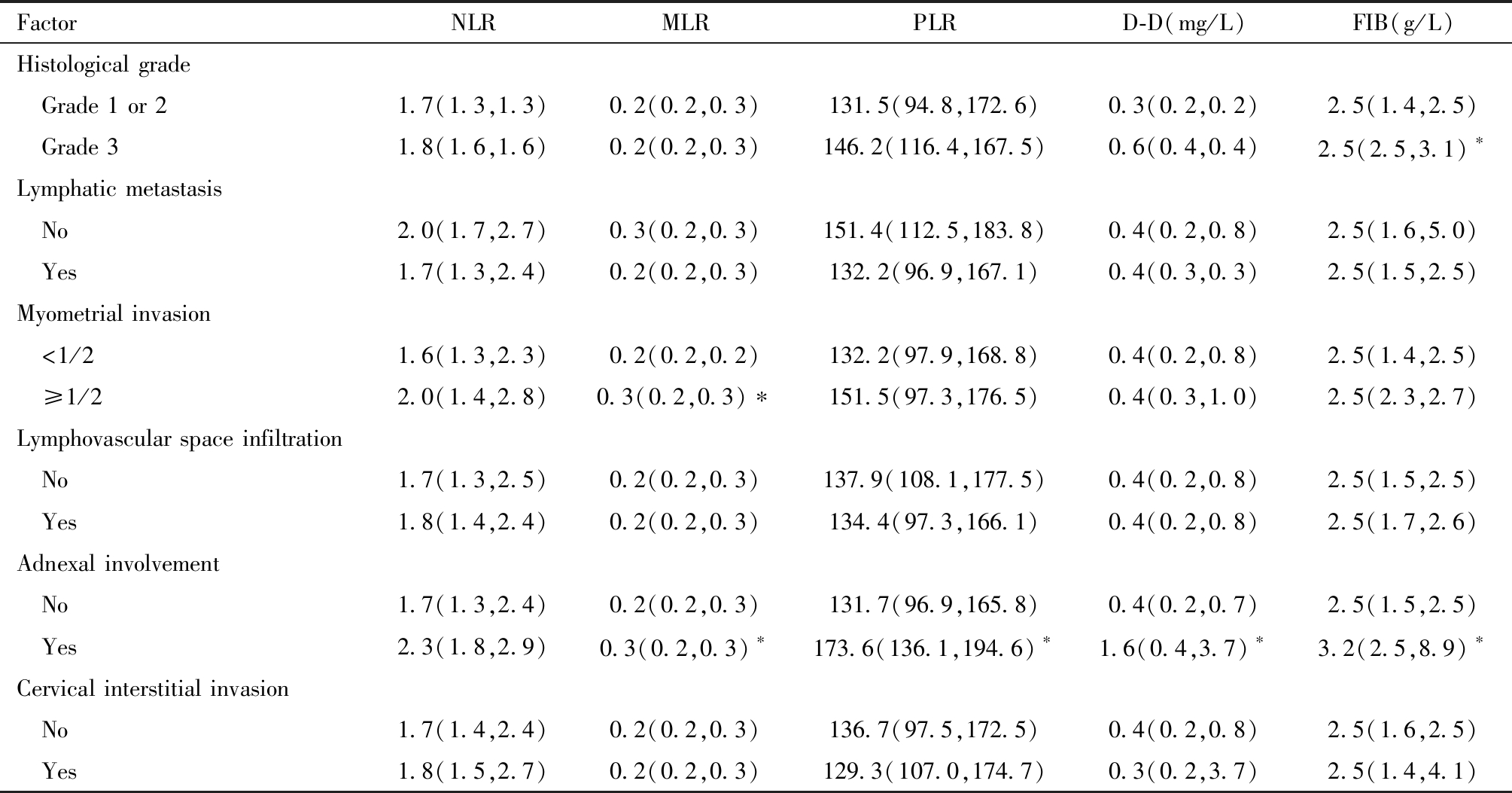
Comparison between the two groups,*P<0.05
FactorNLRMLRPLRD-D(mg/L)FIB(g/L)Histological grade Grade 1 or 21.7(1.3,1.3)0.2(0.2,0.3)131.5(94.8,172.6)0.3(0.2,0.2)2.5(1.4,2.5) Grade 31.8(1.6,1.6)0.2(0.2,0.3)146.2(116.4,167.5)0.6(0.4,0.4)2.5(2.5,3.1)∗Lymphatic metastasis No2.0(1.7,2.7)0.3(0.2,0.3)151.4(112.5,183.8)0.4(0.2,0.8)2.5(1.6,5.0) Yes1.7(1.3,2.4)0.2(0.2,0.3)132.2(96.9,167.1)0.4(0.3,0.3)2.5(1.5,2.5)Myometrial invasion <1/21.6(1.3,2.3)0.2(0.2,0.2)132.2(97.9,168.8)0.4(0.2,0.8)2.5(1.4,2.5) ≥1/22.0(1.4,2.8)0.3(0.2,0.3)∗151.5(97.3,176.5)0.4(0.3,1.0)2.5(2.3,2.7)Lymphovascular space infiltration No1.7(1.3,2.5)0.2(0.2,0.3)137.9(108.1,177.5)0.4(0.2,0.8)2.5(1.5,2.5) Yes1.8(1.4,2.4)0.2(0.2,0.3)134.4(97.3,166.1)0.4(0.2,0.8)2.5(1.7,2.6)Adnexal involvement No1.7(1.3,2.4)0.2(0.2,0.3)131.7(96.9,165.8)0.4(0.2,0.7)2.5(1.5,2.5) Yes2.3(1.8,2.9)0.3(0.2,0.3)∗173.6(136.1,194.6)∗1.6(0.4,3.7)∗3.2(2.5,8.9)∗Cervical interstitial invasion No1.7(1.4,2.4)0.2(0.2,0.3)136.7(97.5,172.5)0.4(0.2,0.8)2.5(1.6,2.5) Yes1.8(1.5,2.7)0.2(0.2,0.3)129.3(107.0,174.7)0.3(0.2,3.7)2.5(1.4,4.1)
三、子宫深肌层浸润与肿瘤直径>2 cm的多因素Logistic回归分析
将上述分析中筛选出的与子宫肌层浸润深度≥1/2与肿瘤直径>2cm有关的临床参数纳入多因素Logistic 回归,分析示血清CA125与CEA是EC患者子宫深肌层浸润的独立影响因素(P<0.05)。血清CA125与HE4是EC患者肿瘤直径>2cm的独立影响因素(P<0.05),见表4及表5。
表4 子宫内膜癌深肌层浸润危险因素的Logistic 回归分析
Table 4 Logistic regression analysis of risk factors for deep myometrial invasion of endometrial cancer
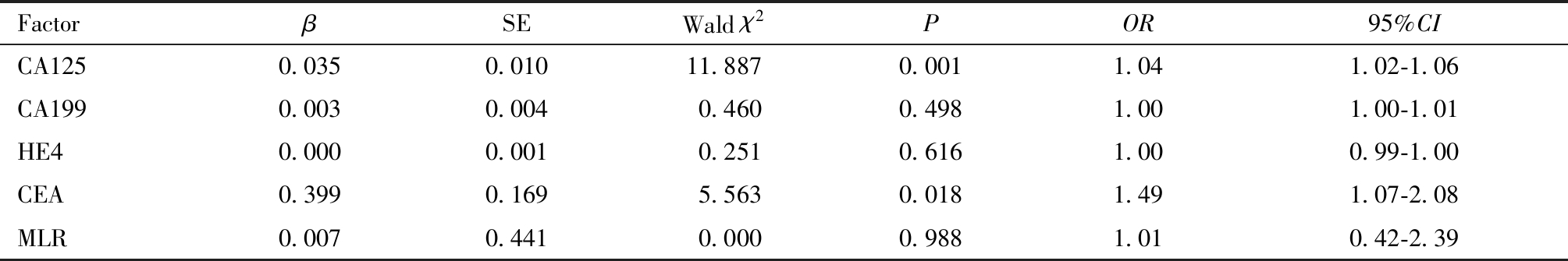
FactorβSEWald χ2POR95%CICA1250.0350.01011.8870.0011.041.02-1.06CA1990.0030.0040.4600.4981.001.00-1.01HE40.0000.0010.2510.6161.000.99-1.00CEA0.3990.1695.5630.0181.491.07-2.08MLR0.0070.4410.0000.9881.010.42-2.39
表5 子宫内膜癌肿瘤直径>2 cm危险因素的Logistic 回归分析
Table 5 Logistic regression analysis of risk factors for endometrial cancer with tumor diameter >2 cm
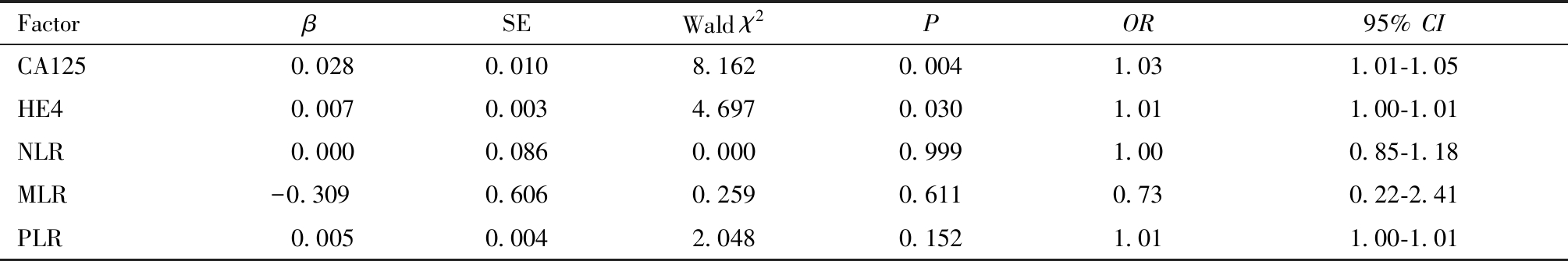
FactorβSEWald χ2POR95% CICA125 0.0280.0108.1620.0041.031.01-1.05HE4 0.0070.0034.6970.0301.011.00-1.01NLR 0.0000.0860.0000.9991.000.85-1.18MLR-0.3090.6060.2590.6110.730.22-2.41PLR 0.0050.0042.0480.1521.011.00-1.01
四、CA125与CEA单独及联合预测EC患者子宫深肌层浸润的效能ROC曲线分析
将CA125、CEA两个参数通过Logistic回归分析,得出联合诊断预测值,绘制ROC曲线,可见CA125与CEA 单独检测均有一定的诊断效能(P<0.05)。联合预测时,CA125+CEA的曲线下面积最大(0.850)。见图1及表6。
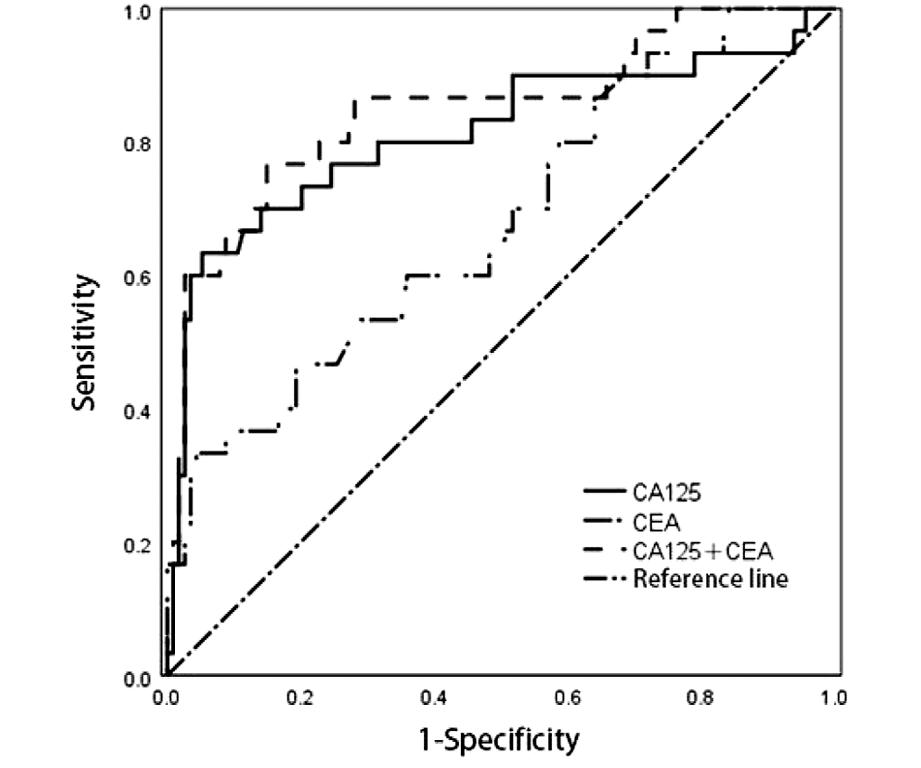
图1 子宫内膜癌患者血清CA125及CEA水平预测评估深肌层浸润的ROC曲线
Figure 1 Shows the ROC curve of serum CA125 and CEA levels in patients with endometrial cancer for predicting and evaluating deep myometrial infiltration
表6 子宫内膜癌患者血清CA125及CEA水平预测评估深肌层浸润的ROC曲线检验结果
Table 6 Results of ROC curve test for predicting and evaluating deep myometrial infiltration by serum CA125 and CEA levels in patients with endometrial cancer
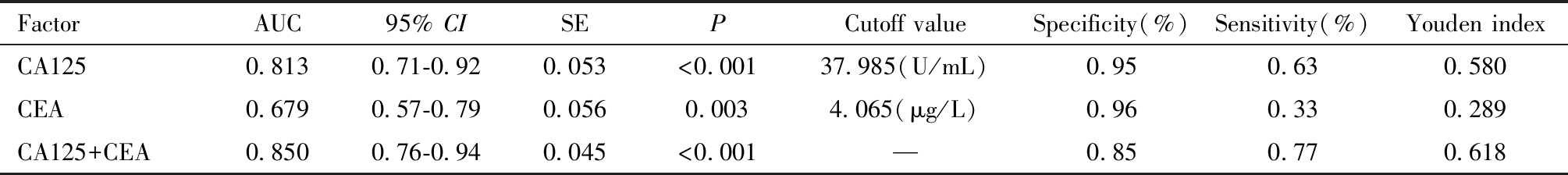
FactorAUC95% CISEPCutoff valueSpecificity(%)Sensitivity(%)Youden indexCA1250.8130.71-0.920.053<0.00137.985(U/mL)0.950.630.580CEA0.6790.57-0.790.0560.0034.065(μg/L)0.960.330.289CA125+CEA0.8500.76-0.940.045<0.001—0.850.770.618
五、CA125与HE4单独及联合预测EC患者肿瘤直径>2 cm的效能ROC曲线分析
将CA125、HE4两个参数通过Logistic回归分析,得出联合诊断预测值,绘制ROC曲线,可见CA125与HE4单独检测均有一定的诊断效能(P<0.05)。联合预测时,CA125+HE4的曲线下面积最大(0.767)。见图2及表7。
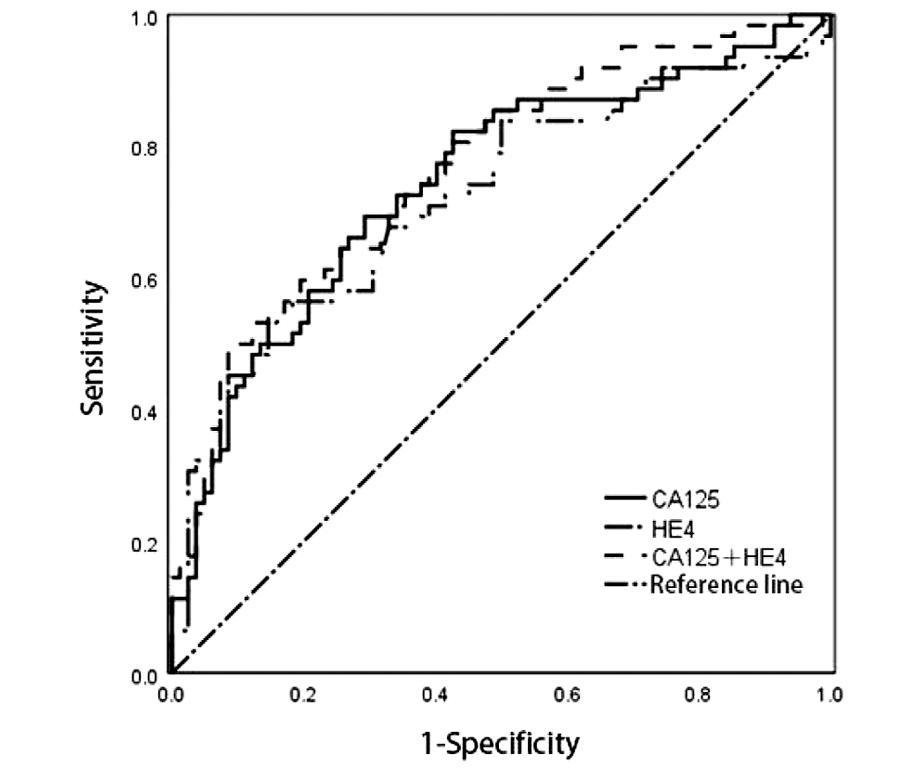
图2 子宫内膜癌患者血清CA125及HE4水平预测评估肿瘤直径>2 cm的ROC曲线
Figure 2 Shows the ROC curves of serum CA125 and HE4 levels in patients with endometrial cancer for predicting and evaluating tumor diameters >2 cm
表7 子宫内膜癌患者血清CA125及HE4水平预测评估肿瘤直径>2 cm的ROC曲线检验结果
Table 7 Results of ROC curve test for predicting and evaluating serum CA125 and HE4 levels in patients with endometrial cancer for tumor diameter >2 cm
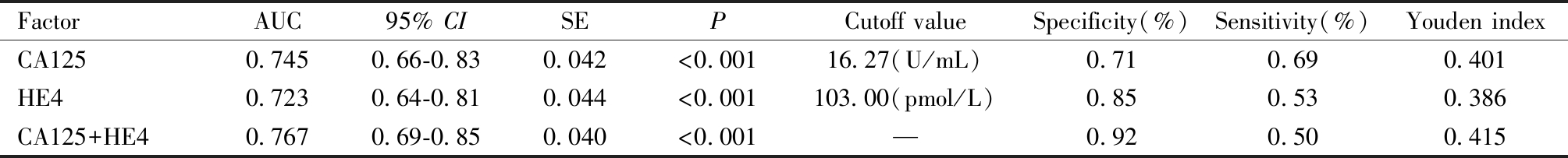
FactorAUC95% CISEPCutoff valueSpecificity(%)Sensitivity(%)Youden indexCA1250.7450.66-0.830.042<0.00116.27(U/mL)0.710.690.401HE40.7230.64-0.810.044<0.001103.00(pmol/L)0.850.530.386CA125+HE40.7670.69-0.850.040<0.001—0.920.500.415
讨 论
EC是女性生殖系统中常见的恶性肿瘤[1]。少数患者由于缺乏特异症状,发现时已处于晚期阶段且预后不佳。手术为EC的主要治疗方法。EC治疗中最大的争议之一是淋巴结清扫术[12]。全面淋巴结清扫术后可能存在多种并发症,如神经损伤、下肢淋巴水肿和盆腔淋巴管膨出,降低患者生活质量[13]。研究报道显示[14],子宫内膜癌侵犯肌层的严重程度与肿瘤直径>2 cm与淋巴结转移率呈正相关,当肿瘤侵犯深肌层、肿瘤直径>2 cm时,淋巴结转移的风险显著增加,故患者需要进行淋巴结清扫;而对于肿瘤浸润深度<1/2,肿瘤直径<2 cm的患者淋巴结转移率<5%,则可能不需行淋巴结清扫术[8];故术前准确评估子宫内膜癌患者肌层浸润情况与肿瘤直径对合理制定手术方案、改善患者预后具有重要意义。
CA125是一种在成人胸膜、心包和腹膜的间充质细胞以及来源于胚胎苗勒氏管的细胞中表达的糖蛋白。此指标在一些健康女性或子宫良性病变中也有不同程度升高。Sun等[15]分析了447例早期子宫内膜癌患者血清CA125水平与预后指出:尽管术前 CA125 在预测初始治疗后早期EC预后的敏感性有限,但它仍然是早期 EC风险评估的有用血清标志物。CEA作为一种结肠癌抗原,是诊断直肠癌的一种生物标志物。已有研究报道血清CEA水平与乳腺癌、卵巢癌、肺癌等多种癌症相关[16]。近年来许多研究表明血清 HE4 与EC预后不良的组织病理特征之间存在关联,包括肿瘤分级、肌层浸润深度≥1/2、肿瘤直径≥2 cm、FIGO分期、宫颈浸润和淋巴结转移 [8,17-18],本研究结果与其一致。现有研究表明CA125、CEA和HE4可增强EC的增殖、侵袭和生长[10,19],可作为EC的诊断、预后和预测性生物标志物。
子宫肌层浸润深度被认为是手术病理分期的重要组成部分,2021年FIGO将子宫肌层浸润<1/2定为IA期,将肿瘤肌层浸润深度≥1/2定为IB期[20]。王娜等[21] 研究报道EC中在深肌层浸润组中CA125与HE4表达水平明显升高,但CEA表达水平在两组间差异不具有统计学意义。而廖旭慧等[10]研究报道CEA与EC组织学分级、淋巴结转移、肌层浸润深度、临床分期均呈正相关。章淑云等[22]通过对120例子宫内膜癌患者分析发现,子宫内膜癌肌层浸润≥1/2组血清CEA水平较浸润深度<1/2组高,且差异具有统计学意义,并强调了血清CEA异常升高是子宫内膜癌发生的危险因素。本研究中子宫内膜癌肌层浸润≥1/2组血清CA125、HE4、CEA水平显著高于肌层浸润<1/2组患者水平,且差异有统计学意义。Prueksaritanond等[7]研究发现HE4 与 EC 的原发肿瘤直径和子宫肌层浸润深度的相关性有助于识别淋巴结转移高风险的EC患者。吴乐策等[14]也证实了EC患者初始病灶越大,淋巴结转移及复发率越高。本研究发现术前血清CA125及HE4水平高的患者肿瘤直径>2 cm的风险就越大。分析原因为随着肌层浸润深度的加深与肿瘤直径的增加,局部侵犯范围增大,导致肿瘤负荷增加,肿瘤细胞聚集的数量增加,因此,血清CA125、HE4、CEA表达增加。高表达的CEA可提高纤维蛋白的结合力使肿瘤侵袭能力增强,且肌层血运丰富,有利于肿瘤细胞获得营养支持,使病情进展[10]。
近年来,已有研究证实单独一项指标在子宫内膜癌早期诊断和预后中灵敏度和特异性均不高,而多指标联合诊断可以提高诊断效能[23]。本研究试图将CA125与CEA进行联合检测,发现EC患者中CA125及CEA异常升高,并且发现联合检测提高了EC患者肌层浸润的诊断效果,将CA125与HE4联合检测提高了EC患者肿瘤直径的诊断效果,准确的诊断生物标志物可以减少因不必要的痛苦和昂贵的检查,因此,CA125、CEA、HE4可尝试用于EC的早期诊断。但本研究为回顾性研究,纳入的样本量相对较小,缺乏多中心数据支持,因此需要今后进一步研究来完善。
1 Crosbie EJ,Kitson SJ,McAlpine JN,et al.Endometrial cancer.Lancet,2022,399:1412-1428.
2 Li J,Wang X,Qu W,et al.Comparison of serum human epididymis protein 4 and CA125 on endometrial cancer detection:A meta-analysis.Clin Chim Acta,2019,488:215-220.
3 Behrouzi R,Barr CE,Crosbie EJ.HE4 as a biomarker for endometrial cancer.Cancers (Basel),2021,13:4764.
4 Stachowicz N,Smoleń A,Ciebiera M,et al.Risk assessment of endometrial hyperplasia or endometrial cancer with simplified ultrasound-based scoring systems.Diagnostics (Basel),2021,11:442.
5 Dueholm M,Hjorth I,Dahl K,et al.Identification of endometrial cancers and atypical hyperplasia:Development and validation of a simplified system for ultrasound scoring of endometrial pattern.Maturitas,2019,123:15-24.
6 Spagnol G,Noventa M,Bonaldo G,et al.Three-dimensional transvaginal ultrasound vs magnetic resonance imaging for preoperative staging of deep myometrial and cervical invasion in patients with endometrial cancer:systematic review and meta-analysis.Ultrasound Obstet Gynecol,2022,60:604-611.
7 Prueksaritanond N,Cheanpracha P,Yanaranop M.Association of serum HE4 with primary tumor diameter and depth of myometrial invasion in endometrial cancer patients at Rajavithi hospital.Asian Pac J Cancer Prev,2016,17:1489-1492.
8 Panyavaranant P,Manchana T.Preoperative markers for the prediction of high-risk features in endometrial cancer.World J Clin Oncol,2020,11:378-388.
9 Saarelainen SK,Peltonen N,Lehtimäki T,et al.Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma.Am J Obstet Gynecol,2013,209:142.e1-6.
10 廖旭慧,张小燕,卢洪胜.癌抗原125 CD68及癌胚抗原在子宫内膜癌患者中的表达与相关性分析.中国妇幼保健,2023,38:3108-3112.
11 王甜甜,张蓓,王晴,等.预后营养指数与MLR、NLR、HE4、CEA、CA125单独及联合检测在子宫内膜癌诊断中的临床价值.徐州医科大学学报,2022,42:725-730.
12 Frost JA,Webster KE,Bryant A,et al.Lymphadenectomy for the management of endometrial cancer.Cochrane Database Syst Rev,2017,10:CD007585.
13 Terada S,Tanaka T,Murakami H,et al.Lymphatic complications following sentinel node biopsy or pelvic lymphadenectomy for endometrial cancer.J Clin Med,2023,12:4540.
14 吴乐策,乔丽娟,袁超群.子宫内膜癌初始病灶大小与淋巴结转移及复发的关系.实用癌症杂志,2023,38:1148-1151.
15 Sun S,Wei L,Zou L,et al.Preoperative serum CA125 level and age at diagnosis:An effective prognosis prediction tool for patients with early-stage endometrial cancer.Asia Pac J Clin Oncol,2023,19:e258-e266.
16 Qiao L,Chen X,Xi X,et al.Correlation analysis and clinical significance of CA125,HE4,DDI,and FDP in type II epithelial ovarian cancer.Medicine (Baltimore),2020,99:e23329.
17 Han LN,Han YW,Yan P.Prognostic values of human epididymis protein 4 expression in patients with endometrial cancer:A systematic review and meta-analysis.J Obstet Gynaecol Res,2022,48:2255-2269.
18 Espiau Romera A,Coronado Martín PJ,Chóliz Ezquerro M,et al.Value of preoperative HE4 as predictor of advanced disease in endometrioid endometrial cancer.Int J Gynaecol Obstet,2021,153:64-70.
19 Lu Q,Chen H,Senkowski C,et al.Recombinant HE4 protein promotes proliferation of pancreatic and endometrial cancer cell lines.Oncol Rep,2016,35:163-170.
20 Ozdemir CY,Telli EU,Oge T et al.Ultrasonography,macroscopy,and frozen section:wh ch is better for predicting deep myometrial invas
ch is better for predicting deep myometrial invas on in endometrial cancer?.Rev Assoc Med Bras (1992),2023,69:e20230333.
on in endometrial cancer?.Rev Assoc Med Bras (1992),2023,69:e20230333.
21 王娜,郭云峰,宋立芹,等.HE4、CA125及CA199在鉴别子宫内膜癌肌层浸润深度及病理分期中的临床应用价值.重庆医科大学学报,2022,47:1220-1225.
22 章淑云,倪正亚,颜雅萍.血清CEA、CA153、CA72-4联合检测诊断子宫内膜癌价值.中国计划生育学杂志,2023,31:1469-1473.
23 Barr CE,Njoku K,Jones ER,et al.Serum CA125 and HE4 as biomarkers for the detection of endometrial cancer and associated high-risk features.Diagnostics (Basel),2022,12:2834.