新生儿呼吸窘迫综合征(neonatal respiratory distress syndrome,NRDS)为肺表面活性物质(pulmonary surfactant,PS)缺乏所致,多见于早产儿,补充外源性PS是RDS病因治疗的主要方法。采用PS及机械通气治疗,RDS早产儿易出现短期呼吸系统合并症,包括肺出血、气漏、呼吸机相关性肺炎和支气管肺发育不良等。15年前首次提出了以PS为载体气管内滴入药物的概念[1],近年来一些研究表明,以PS为载体气管内滴入布地奈德可以减少PS消耗及促进PS的合成和再循环,有效治疗RDS;此外,由于PS 作为蛋白载体增加了布地奈德混悬液的溶解和吸收,提高了气道选择性和抗炎作用,从而减轻肺损伤及全身炎症反应 [2-5]。本文对出生后早期以PS为载体气管内滴入布地奈德治疗早产儿RDS进行回顾性研究,探讨其改善RDS早产儿短期呼吸系统合并症的有效性,并积累相关临床治疗经验。
对象与方法
一、对象
对象来源于北京大学第三医院NICU 2014年1月1日至2018年12月31日出生后1 h内收入新生儿,纳入标准:(1)胎龄26~32 周;(2)生后呼吸困难进行性加重,诊断为RDS;(3)生后早期应用猪肺磷脂注射液或猪肺磷脂注射液+布地奈德混悬液。排除标准:(1)呼吸系统发育畸形、严重的先天缺陷和严重的先天性心脏病者;(2)住院期间放弃治疗及转外院治疗者。分为PSBu组和PS组,PSBu组:气管内滴入PS(猪肺磷脂注射液,意大利凯西制药公司生产,规格为240 mg/3 mL)和布地奈德混悬液(AstraZeneca Pty Ltd生产,规格为1 mg/2 mL)混合剂 (每150~200 mg/kg PS中加入0.25 mg/kg布地奈德混悬液);PS组:气管内单独滴入PS(150~200 mg/kg)。
二、方法
1.资料搜集:通过本院病案科病历档案室和电子病例系统查询符合入组标准的病例,设计临床调查表,记录患儿临床基本信息、RDS治疗情况、短期呼吸系统合并症(肺出血、气漏、呼吸机相关性肺炎、支气管肺发育不良)发生率及其他合并症和存活率等。
2.疾病诊断标准:参考《实用新生儿学》第4版[6],包括RDS、肺出血、气漏、呼吸机相关性肺炎、支气管肺发育不良、动脉导管未闭、脑室内出血、脑室周围白质软化、坏死性小肠结肠炎、晚发败血症、早产儿视网膜病。其中 RDS指生后出现进行性呼吸困难,根据胸片改变分为4级,即I级指两肺野普遍透亮度降低,可见细颗粒及网状阴影;II级指除I级变化加重外,可见支气管充气征,延伸至肺野中外带;III级指肺野透亮度更加减低,心缘、膈缘模糊;IV级呈“白肺”。支气管肺发育不良是指任何氧依赖超过28 d的新生儿,胎龄<32周时根据矫正胎龄36周或出院时需FiO2分为轻度、中度和重度,轻度指未用氧,中度指FiO2<30%,重度指FiO2≥30% 或需机械通气。采用超声心动图检查诊断动脉导管未闭;应用头颅影像学诊断脑室内出血和脑室周围白质软化,脑室内出血分级采用Papile分级法;坏死性小肠结肠炎分期采用Bell分期法。
3.统计学处理:应用SPSS 23.0软件进行统计分析,正态分布的计量资料采用![]() 表示;组间均数比较采用独立样本t检验;计数资料采用频数及百分率表示,组间比较应用χ2检验或Fisher精确概率法;以P<0.05为差异有统计学意义。
表示;组间均数比较采用独立样本t检验;计数资料采用频数及百分率表示,组间比较应用χ2检验或Fisher精确概率法;以P<0.05为差异有统计学意义。
结 果
一、临床基本信息
符合入组标准128例,PSBu组共61例,PS组共67例,两组在出生体重、胎龄、男婴、产前未用激素、剖宫产、宫内窘迫、1 min Apgar评分和RDS分级比较,差异均无统计学意义,具有可比性,见表1。
二、RDS治疗情况
PSBu组PS重复给药次数较PS组少,但组间差异没有统计学意义;PSBu组无创通气和总用氧时间均小于PS组,差异有统计学意义;两组有创通气时间差异无统计学意义。见表2。
三、合并症
PSBu组呼吸机相关性肺炎发生率明显低于PS组,支气管肺发育不良(轻-中度)发生率显著低于PS组,差异均有统计学意义;两组患儿肺出血、气漏及其他合并症发生率差异均无统计学意义。见表3。
四、转归
PSBu组存活率为90.2%(56/61),PS组存活率为89.6%(60/67),两组存活率比较,差异无统计学意义。
表1 PSBu组与PS组临床基本信息比较
Table 1 Comparison of basic clinical information between group of PSBu and PS
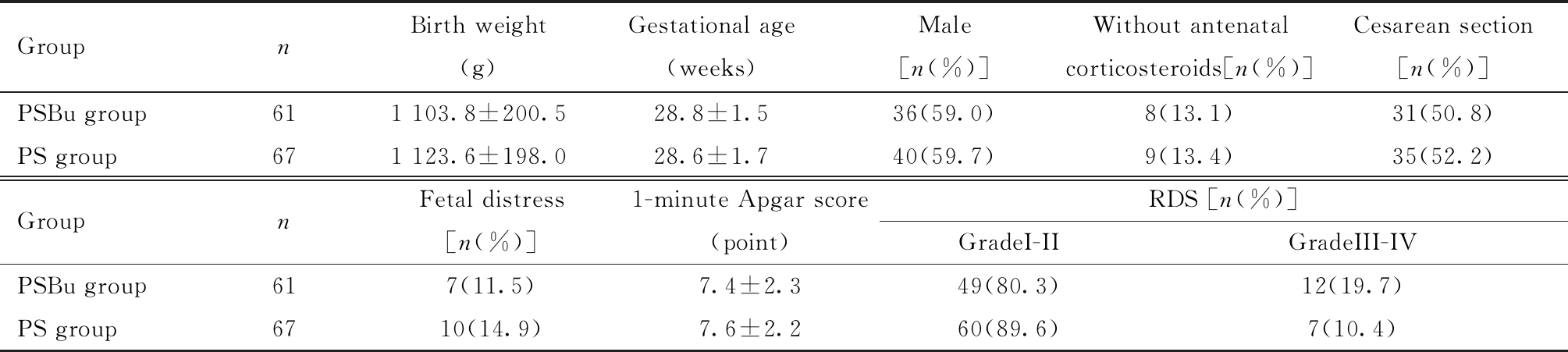
GroupnBirth weight(g)Gestational age(weeks)Male[n(%)] Without antenatal corticosteroids[n(%)]Cesarean section[n(%)]PSBu group611 103.8±200.528.8±1.536(59.0) 8(13.1)31(50.8)PS group671 123.6±198.028.6±1.740(59.7) 9(13.4)35(52.2)GroupnFetal distress[n(%)]1-minute Apgar score(point)RDS [n(%)]GradeI-IIGradeIII-IVPSBu group617(11.5)7.4±2.349(80.3)12(19.7)PS group6710(14.9)7.6±2.260(89.6)7(10.4)
GroupPSBu=Pulmonary surfactant (150-200 mg/kg) + Budesonide (0.25 mg/kg); Group PS=Pulmonary surfactant (150-200 mg/kg); RDS=respiratory distress syndrome
表2 PSBu组与PS组RDS治疗情况比较![]() 或例(%)]
或例(%)]
Table 2 Comparison of RDS treatment between group of PSBu and PS ![]() , n(%)]
, n(%)]

GroupnFirst dose ofsurfactant(mg/kg)Frequency of Surfactant administration1 dose>1 doseDays of Invasive mechanical ventilation(d)Days of Noninvasive mechanical ventilation(d)Days of Supplemental oxygen(d)PSBu group61195.6±9.556(91.8)5(8.2)3.9±0.823.3±7.7*36.6±12.9*PS group67192.9±12.257(85.1)10(14.9)4.3±1.227.8±8.638.5±13.7
GroupPSBu=Pulmonary surfactant (150-200 mg/kg) + Budesonide (0.25 mg/kg); Group PS=Pulmonary surfactant (150-200 mg/kg); Compared with PS group,*P<0.05
表3 PSBu组与PS组合并症比较[例(%)]
Table 3 Comparison of complications between group of PSBu and PS [n(%)]
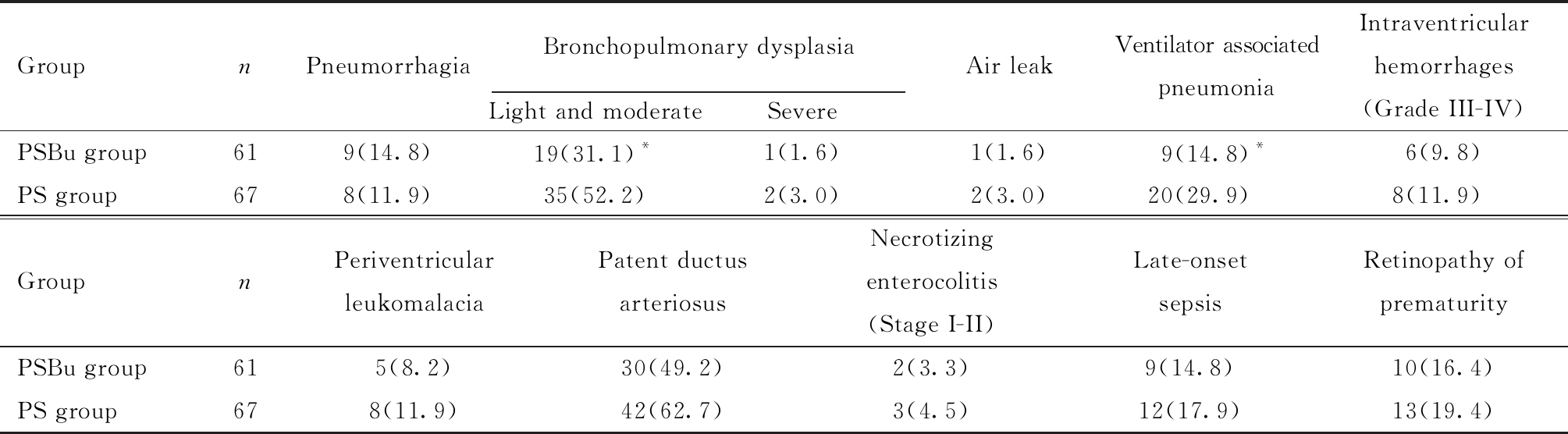
GroupnPneumorrhagiaBronchopulmonary dysplasiaLight and moderate SevereAir leakVentilator associatedpneumoniaIntraventricular hemorrhages(Grade III-IV)PSBu group619(14.8)19(31.1)*1(1.6)1(1.6)9(14.8)*6(9.8)PS group678(11.9)35(52.2)2(3.0)2(3.0)20(29.9)8(11.9)GroupnPeriventricular leukomalaciaPatent ductus arteriosusNecrotizingenterocolitis (Stage I-II)Late-onset sepsisRetinopathy of prematurityPSBu group615(8.2)30(49.2)2(3.3)9(14.8)10(16.4)PS group678(11.9)42(62.7)3(4.5)12(17.9)13(19.4)
Group PSBu=Pulmonary surfactant (150-200 mg/kg) + Budesonide (0.25 mg/kg); Group PS=Pulmonary surfactant (150-200 mg/kg); Compared with PS group,*P<0.05
讨 论
PS被常规用于治疗早产儿RDS,应用PS及机械通气后难免对不成熟的肺造成了机械性损伤和高氧损伤,损伤后的肺组织容易受到病原菌入侵,导致细胞因子介导的炎性反应瀑布式发生,肺组织炎性渗出,从而发生感染性肺炎,肺内炎症反应、肺损伤及高氧损伤进一步导致支气管肺发育不良的发生[7]。布地奈德局部抗炎作用较强而全身不良反应较小[8],以PS为载体气管内滴入布地奈德后,由于肺表面梯度张力导致PS在肺内迅速弥散,并有助于布地奈德混悬液弥散至远端支气管和肺泡,从而起到局部抗炎和扩张支气管作用;进入肺泡组织的布地奈德与肺泡II型细胞受体特异性结合后会产生多种激素相关蛋白,并作用于肺泡Ⅱ型细胞促进 PS的合成和释放,也能够降低肺微血管的通透性和减少肺水肿,从而改善肺功能[9]。
本研究回顾性比较PSBu组(PS联合布地奈德)与PS组两组患儿,发现PSBu组显著缩短了无创通气和总用氧时间,降低了呼吸机相关性肺炎和轻-中度支气管肺发育不良发生率,差异均具有统计学意义。一项临床研究表明,布地奈德随着PS到达整个肺组织后可以抑制瀑布式炎性反应;与单纯应用PS相比,PS联合布地奈德治疗早产儿RDS,在用药后12 h内气管分泌物中白细胞介素-1、白细胞介素-6、白细胞介素-8等炎症因子水平明显减低,在3~5 d和7~8 d内白细胞介素-8明显降低,明显减轻肺部炎症,降低支气管肺发育不良的发生率[10]。Yang等[11]建立的PS缺乏的小猪模型中研究表明,气管内滴入PS联合布地奈德(100 mg/kg PS + 0.25 mg/kg布地奈德)治疗严重RDS,与单纯应用PS(100 mg/kg)相比,可明显改善氧合,导致肿瘤坏死因子-α和白细胞介素-1 β下降,减轻肺部炎症,明显改善肺功能。给予机械通气的早产羊气管内滴入PS联合布地奈德混合物,与仅气管内滴入PS相比,在最初24 h内需要更低的平均气道压、更低的氧浓度和更稳定的血压,肺、肝和脑的炎症因子明显减低,诱导型一氧化氮合酶活性降低,明显改善肺功能,减轻肺损伤及全身炎症反应[12]。Yeh等[13]将生后发生严重RDS(即生后4 h内需要机械通气,吸入氧浓度>60%,除外先天性心肺疾病)的早产儿早期应用PS联合布地奈德组拔管时间比PS组提前1~2周。本研究中两组有创通气使用时间差异无统计学意义,可能与入组患儿RDS多为I~II级,应用有创通气时间较短有关。
本研究中两组气漏和肺出血差异没有统计学意义。此外,III~IV级脑室内出血、脑室周围白质软化、动脉导管未闭、坏死性小肠结肠炎(I-II期)、晚发败血症和早产儿视网膜病的发生率及存活率差异没有统计学意义,提示以PS为载体气管内滴入布地奈德不增加其他疾病和死亡的风险。近年来,研究表明,布地奈德联合PS气管内滴入治疗早产儿RDS不仅降低了支气管肺发育不良发生率,而且未增加死亡率及神经系统预后不良的风险[13-14]。在一项2~3年的随访研究中也表明,气管内滴入布地奈德联合PS未发现对生长发育及神经发育造成不良影响。气管内滴入布地奈德没有引起直接或远期的全身副作用,这可能是因为滴入气管内的布地奈德可逆地与脂肪酸结合形成布地奈德酯,可以逐步释放布地奈德,布地奈德没有在肺细胞内代谢,充分发挥了其抗炎作用;血中布地奈德半衰期是4.13 h,小于绝大多数药物,在肝脏和其他组织中很快代谢,故低剂量的布地奈德在局部应用是有效的,且代谢产物的全身副作用非常小 [15]。
综上所述,将PS作为载体,气管内滴入布地奈德,缩短了RDS早产儿无创通气和总吸氧时间,降低了呼吸机相关性肺炎和轻-中度支气管肺发育不良发生率,部分改善了短期呼吸系统合并症,气漏和肺出血无明显降低,未增加其他合并症发生率和死亡率。本研究为单中心回顾性研究,尚需在未来的临床应用中对药物剂量、给药频率、呼吸机模式等进行大样本、多中心的对照研究,并在动物实验中对改善肺功能的相关分子机制做进一步研究。
1 van′t Veen A,Gommers D,Mouton JW,et al.Exogenous pulmonary surfactant as a drug delivering agent:influence of antibiotics on surfactant activity.Br J Pharmacol,1996,118:593-598.
2 Bassler D.Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.Neonatology,2015,107:358-359.
3 陈奕江,张水堂,杨润娜.生后7天内气道内应用布地奈德对早产儿支气管肺发育不良发生率影响的系统评价和Meta分析.中国循证儿科杂志,2016,11:258-264.
4 Ricci F,Catozzi C,Ravanetti F,et al.In vitro and in vivo characterization of poractant alfa supplemented with budesonide for safe and effective intratracheal administration.Pediatr Res,2017,82:1056-1063.
5 Roberts JK,Stockmann C,Dahl MJ,et al.Pharmacokinetics of Budesonide Administered with Surfactant in Premature Lambs:Implications for Neonatal Clinical Trials.Curr Clin Pharmacol,2016,11:53-61.
6 邵肖梅,叶鸿瑁,丘小汕.实用新生儿学.北京:人民卫生出版社,第4版,2011:395-887.
7 Surate Solaligue DE,Rodríguez-Castillo JA,Ahlbrecht K,et al.Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol,2017,13:L1101-L1153.
8 Hvizdos KM,Jarvis B.Budesonide inhalation suspension:a review of its use in infants,children and adults with inflammatory respiratory disorders.Drugs,2000,60:1141-1178.
9 Gupta S,Prasanth K,Chen CM,et al.Postnatal corticosteroids for prevention and treatment of chronic lung disease in the preterm newborn.Int J Pediatr,2012,2012:315642.
10 Yeh TF,Lin HC,Chang CH,et al.Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants:a pilot study.Pediatrics,2008,121:e1310-1318.
11 Yang CF,Lin CH,Chiou SY,et al.Intratracheal budesonide supplementation in addition to surfactant improves pulmonary outcome in surfactant-depleted newborn piglets.Pediatr Pulmonol,2013,48:151-159.
12 Kothe TB,Kemp MW,Schmidt AF,et al.Surfactant plus budesonide decreases lung and systemic inflammation in mechanically ventilated preterm sheep.Am J Physiol Lung Cell Mol Physiol,2019,316:L888-L893.
13 Yeh TF,Chen CM,Wu SY,et al.Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia.Am J Respir Crit Care Med,2016,193:86-95.
14 Venkataraman R,Kamaluddeen M,Hasan SU,et al.Intratracheal Administration of Budesonide-Surfactant in Prevention of Bronchopulmonary Dysplasia in Very Low Birth Weight Infants:A Systematic Review and Meta-Analysis.Pediatr Pulmonol,2017,52:968-975.
15 Kuo HT,Lin HC,Tsai CH,et al.A follow-up study of preterm infants given budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants.J Pediatr,2010,156:537-541.