·妇幼保健·
术前NLR、PLR和CA125水平对子宫内膜癌预后的评估价值
高敏 王巍 宋楠 张乃怿 郑虹 高雨农
作者单位:100142 北京,北京大学肿瘤医院(暨北京市肿瘤防治研究所)妇科,恶性肿瘤发病机制及转化研究教育部重点实验室
通讯作者:高雨农(gaoyunong@vip.sina.com)
【摘要】 目的 探讨子宫内膜癌患者术前外周血中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)和血清CA125水平与子宫内膜癌预后的关系。方法回顾性分析2010年10月至2013年11月期间在北京大学肿瘤医院妇科接受治疗的145例子宫内膜癌患者的临床资料及随访结果,患者年龄30~77岁,平均年龄(53.4±7.9)岁,采用FIGO 2009年的子宫内膜癌手术病理分期标准确定分期。测定术前1周内的血常规和血清CA125水平,计算出NLR和PLR值,绘制受试者工作特性(ROC)曲线,通过约登指数最大值确定预测复发和死亡的最佳临界值。通过单因素和多因素分析评估NLR、PLR和CA125对子宫内膜癌预后的影响。结果III/IV期子宫内膜癌患者和淋巴结转移患者的NLR、PLR和CA125水平明显高于I/II期和无淋巴结转移患者。多因素分析显示,NLR、病理分级和淋巴结转移是无进展生存期(PFS)的独立预后因素;NLR和组织学类型是OS的独立预后因素。而PLR和CA125水平变化没有显示出对预后的明确预测价值。结论术前NLR值测定对评估子宫内膜癌预后具有很好的临床价值,高NLR值是子宫内膜癌预后不良的独立预测因素。
【关键词】 子宫内膜癌; 中性粒细胞与淋巴细胞比值(NLR); 血小板与淋巴细胞比值(PLR); 糖类抗原125(CA125); 预后
The prognostic value of preoperative NLR, PLR andserum CA125 levels in endometrial carcinoma
GAO Min, WANG Wei, SONG Nan, ZHANG Naiyi, ZHENG Hong, GAO Yunong.
Key laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of gynecologic oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
[Abstract] Objective To investigate the relationship between preoperative peripheral blood neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and serum CA125 level with prognosis of endometrial carcinoma.MethodsA retrospective analysis of 145 patients with endometrial cancer who were treated in the Department of Gynecology of Peking University Cancer Hospital between Oct 2010 and Nov 2013 was performed. The mean age of patients was (53.4±7.9) years (range 30-77 years). The surgical staging of endometrial cancer was determined according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 guidelines. We assessed values of preoperative NLR, PLR and serum CA125. Receiver operating characteristic (ROC) curves were plotted and the optimal cut-off values of NLR, PLR and CA125 were calculated for predicting recurrence and death. Univariate and multivariate analyses were performed to assess the effect of preoperative NLR, PLR and CA125 on the prognosis of endometrial cancer.ResultsThe levels of NLR, PLR, and CA125 in patients with stage III/IV endometrial cancer and lymph node metastasis were significantly higher than those with stage I/II and no lymph node metastasis. Multivariate analysis showed that NLR, pathological grade and lymph node metastasis were independent prognostic factors for progression-free survival (PFS). NLR and histologic type are independent prognostic factors for overall survival (OS). However, PLR and CA125 levels did not show the predictive value for prognosis.ConclusionPreoperative NLR value has a good clinical value in evaluating the prognosis of endometrial cancer, and high NLR value is an independent predictor of poor prognosis in endometrial cancer.
Key words] endometrial cancer; neutrophil to lymphocyte ratio; platelet to lymphocyte ratio; carbohydrate antigen 125; prognosis
子宫内膜癌是发达国家女性生殖系统最常见的恶性肿瘤。中国的发病率近年来也逐渐升高,北京、上海等发达城市子宫内膜癌的发病率已超过宫颈癌,成为妇科恶性肿瘤中发病率最高的肿瘤。近年来,研究证实,肿瘤的发生与机体的免疫炎症反应关系密切。中性粒细胞增多、淋巴细胞减少和血小板增多是最常见的全身性改变[1-2]。系统性炎症反应(SIR),如淋巴细胞减少,导致中性粒细胞与淋巴细胞比值(NLR)增加,被认为是肿瘤进展和侵袭的机制之一[3-5]。一些研究表明,NLR、血小板与淋巴细胞比值(platelet/lymphocyte ratio, PLR)可能与肺癌、乳腺癌、胰腺癌和非小细胞肺癌等实体肿瘤的不良预后有关[6-9]。然而,有关系统性炎症反应对子宫内膜癌预后的影响研究较少。本研究旨在通过回顾性分析探讨NLR、PLR和CA125对子宫内膜癌预后的评估价值。
资料与方法
一、一般资料
回顾性分析2010年10月至2013年11月在北京大学肿瘤医院接受治疗的145例子宫内膜癌患者的临床资料。所有患者均接受了手术治疗,手术范围包括全子宫切除术、双侧附件切除术、盆腔和/或腹主动脉旁淋巴结切除术。采用FIGO 2009年的子宫内膜癌手术病理分期标准确定分期[10]。临床病理资料包括患者年龄、分期、组织学类型、病理分级、肌层浸润深度、淋巴结转移、淋巴脉管间隙受累(LVSI)、复发和生存时间等。
患者年龄30~77岁,平均年龄(53.4±7.9)岁。 145例患者中有118例 (81.4%) 组织学类型为 I型子宫内膜癌,27例 (18.6%) 组织学类型为II 型子宫内膜癌。 FIGO分期I/II期患者121例,占83.4%;FIGO III/IV 期24例,占16.6%。145例患者中,病理分级为1级和2级者总计97例,占66.9%,病理分级为3级者48例,占33.1%。
二、方法
1. 检查方法及随访情况:所有患者于术前1周内行血常规及血清CA125检查,根据血常规结果计算NLR和PLR。 NLR定义为中性粒细胞数与淋巴细胞数比值,PLR定义为血小板数与淋巴细胞数比值。依据术前NLR值绘制受试者工作特性(ROC)曲线,通过约登指数最大值确定预测复发和死亡的最敏感临界值(cut-off值)。患者在术后前两年每3个月随访一次,术后3~5年每6个月随访一次。
2. 统计学处理:采用SPSS17.0软件进行统计学分析。描述性分析采用均数±标准差,分类变量采用Mann-Whitney u检验。生存分析采用Kaplan-Meier法检验,预后危险因素采用Cox回归模型。通过ROC曲线分析和计算NLR、PLR和血清CA125预测复发和转移的最佳临界值。P<0.05为差异有统计学意义。
结 果
一、不同病理特征下子宫内膜癌患者NLR、PLR和CA125的水平变化及临床意义
如表1所示,III/IV期子宫内膜癌患者的NLR、PLR和CA125水平均显著高于I/II期子宫内膜癌患者,淋巴结转移患者的NLR、PLR和CA125水平也明显高于无淋巴结转移患者,差异均有统计学意义。同时,存在脉管癌栓和深肌层浸润的患者PLR和CA125水平高于无脉管癌栓和浅肌层浸润者,但NLR值差异未见统计学意义。
表1 不同病理特征下的NLR、PLR和CA125水平
Table 1 The levels of NLR, PLR and CA125 according
to the different pathological characteristics

VariableNLRPLRCA125(U/mL)Histopathologic type Type Ⅰ1.93±1.04131.71±57.5928.97±40.60 Type Ⅱ2.11±1.11132.17±30.4849.65±114.68Stage Ⅰ-Ⅱ1.83±0.92127.18±49.1932.17±65.36 Ⅲ-Ⅳ2.72±1.40∗157.65±69.53∗36.09±36.85∗Grade G1-21.91±0.97129.31±50.9233.59±70.51 G32.08±1.21136.95±59.0431.27±37.71Myometrial invasion <1/21.85±0.93126.38±49.2327.54±58.19 ≥1/22.32±1.33148.74±63.30∗49.40±69.13∗LVSI Negative1.91±1.02126.93±49.9628.57±61.23 Positive2.17±1.17151.66±63.68∗47.82±60.93∗Lymph node metastasis Negative1.84±0.91126.67±48.0832.00±63.50 Positive3.18±1.58∗183.05±77.76∗39.94±40.77∗
Compared between the two group, *P<0.05
二、影响子宫内膜癌患者无进展生存期(progression-free survival,PFS)的因素分析
单因素分析显示,NLR、组织学类型、病理分级、分期、肌层浸润深度、LVSI和淋巴结转移是PFS的影响因素。然而多因素分析显示,只有NLR、病理分级和淋巴结转移是PFS的独立预后因素。如表2所示。
表2 不同参数特征下的子宫内膜癌患者PFS的单因素和多因素分析
Table 2 Univariate and multivariate analysis for PFS of patients
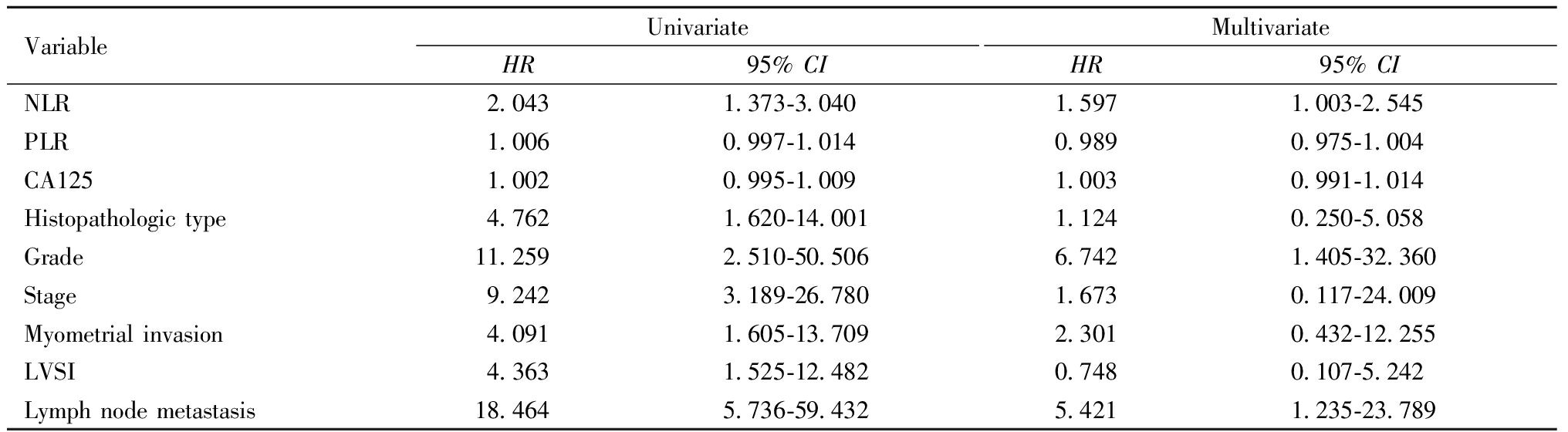
VariableUnivariateHR95% CI MultivariateHR95% CI NLR2.0431.373-3.0401.5971.003-2.545PLR1.0060.997-1.0140.9890.975-1.004CA1251.0020.995-1.0091.0030.991-1.014Histopathologic type4.7621.620-14.0011.1240.250-5.058Grade11.2592.510-50.5066.7421.405-32.360Stage9.2423.189-26.7801.6730.117-24.009Myometrial invasion4.0911.605-13.7092.3010.432-12.255LVSI4.3631.525-12.4820.7480.107-5.242Lymph node metastasis18.4645.736-59.4325.4211.235-23.789
三、影响子宫内膜癌患者总生存期(overall survival, OS)的因素分析
单因素分析显示,NLR、组织学类型、病理分级、分期、肌层浸润深度和淋巴结转移是OS的影响因素。然而多因素分析显示,只有NLR和组织学类型是OS的独立预后因素。如表3所示。
表3 不同参数特征下的子宫内膜癌患者OS的单因素和多因素分析
Table 3 Univariate and multivariate analysis for OS of patients

VariableUnivariateHR95% CI MultivariateHR95% CI NLR2.2441.422-3.5422.5751.500-4.423PLR1.0080.997-1.0111.0040.984-1.024CA1251.0020.994-1.0110.9910.951-1.034Histopathologic type10.2711.979-53.32110.7561.750-66.108Grade11.5641.388-96.3071.4280.991-2.703Stage7.7501.723-34.8511.5720.221-26.378Myometrial invasion8.9991.737-46.6142.3280.300-18.075LVSI2.8900.644-12.9680.3040.015-6.150Lymph node metastasis15.6883.366-73.120— —
四、NLR值与子宫内膜癌预后的关系
通过约登指数计算出预测PFS和OS的cut-off值分别为NLR≥2.46和NLR≥2.56,依据此数值将患者分为高NLR组和低NLR组。结果显示,高NLR组5年PFS为73.1%,而低NLR组的5年PFS为89.1%,两组差异有统计学意义。高NLR组5年总生存率为83.3%,而低NLR组5年总生存率为90.9%,两组差异有统计学意义。如图1、图2。
讨 论
中性粒细胞/淋巴细胞(NLR)、血小板计数/淋巴细胞(PLR)比值反映了机体炎症反应与免疫状态之间的平衡关系,而慢性炎症反应和机体免疫状态与肿瘤的发生、发展关系密切[11-12]。其比值的变化在很多恶性肿瘤中如结直肠癌、乳腺癌、胃癌、胰腺癌、膀胱癌等已证实与肿瘤的预后相关[13-17]。同时,NLR在不同肿瘤的术前诊断方面也具有很好的预测价值。Sahin等[18]回顾性分析140例肺癌患者的资料,发现NLR值在肺癌患者中明显高于正常人群,NLR诊断临界值为1.5,灵敏度为86%,特异度达92%,发生转移的肺癌患者NLR值更高。 Laohawiriyakamol等[15]报道高NLR比值是乳腺癌患者淋巴结转移很好的预测因子。 HanByoul等[19]在卵巢癌的研究中显示,术前NLR 比值与卵巢上皮性癌的预后具有明确的相关性,当NLR大于 2.6时患者的预后较差。 NLR、PLR比值可通过血液学检查获得,方法简单,无创伤性,因此,在术前对恶性肿瘤患者预后的评估具有很大的优势,但是在子宫内膜癌中的研究资料有限。一项对197例子宫内膜癌患者的回顾性研究表明,单因素分析显示NLR和PLR是淋巴结转移的预测因子,NLR和PLR的最佳临界值分别为2.18和206。然而多因素分析显示仅NLR是淋巴结转移的独立预测因子[20]。Haruma等[21]研究显示,高NLR组的子宫内膜癌患者无疾病生存期(DFS)和OS较低NLR组明显缩短,评估DFS的NLR值为2.41,评估OS的NLR值为2.71;多因素分析显示NLR是影响预后的独立预测因子。本研究回顾性分析了145例子宫内膜癌患者术前1周内的血常规结果,计算出NLR和PLR值,通过约登指数最大值计算出评估PFS和OS的最佳临界值,结果显示,仅NLR是影响患者PFS和OS的独立预测因子,而PLR和CA125水平变化没有显示出对预后的明确预测价值。在评估PFS时,NLR的最佳临界值为2.46,高NLR组患者的PFS较低NLR组明显缩短。在评估OS时,NLR的最佳临界值为2.56,高NLR组患者的OS明显短于低NLR组。
尽管在多种恶性肿瘤中均发现NLR值与疾病的预后密切相关,但确切的机制尚不清楚。可能的机制为NLR升高是由于中性粒细胞的增多和淋巴细胞的减少共同导致。肿瘤组织释放的多种细胞因子可以刺激中性粒细胞升高,而中性粒细胞的升高又为肿瘤的发生发展提供合适的微环境。传统免疫学上中性粒细胞主要负责机体的防御和免疫调节。但在肿瘤患者体内,中性粒细胞的功能出现多样化。一方面,中性粒细胞表现为防御作用;另一方面,肿瘤微环境中的各种刺激因子导致部分中性粒细胞活化为促肿瘤活性。中性粒细胞的增多还可以抑制T淋巴细胞的活化,而作为机体重要的免疫防御作用的淋巴细胞其主要功能是抑制肿瘤的生长,促进肿瘤细胞的凋亡,淋巴细胞的减少将导致机体的免疫功能出现异常。因此,中性粒细胞和淋巴细胞的变化是机体免疫状态失衡的表现。而这种免疫失衡状态在外周血中表现为中性粒细胞和淋巴细胞比值即NLR值的改变。当NLR比值升高时预示机体的免疫功能减低,对肿瘤的抑制作用减弱,导致预后不良[22-23]。
总之,外周血中NLR值在一定程度上反映了机体的免疫状态,对预测肿瘤的预后具有很好的指导价值。同时NLR值获取方便,仅于术前行血常规检测即可,方便实用,价格低廉,是目前最为简便且无创性的检查方法,值得在临床推广应用。由于本研究样本例数有限,且为回顾性研究,尚需要大样本、前瞻性研究进一步加以验证。
参考文献
1 Hanahan D,Weinberg R.Hallmarks of cancer:the next generation.Cell,2011,144:646-674.
2 Grivennikov SI,Greten FR,Karin M.Immunity,inflammation,and cancer.Cell,2010,140:883-899.
3 Roxburgh CS,McMillan DC.Role of systemic inflammatory response in predicting survival in patients with primary operable cancer.Future Oncol,2010,6:149-163.
4 Allavena P,Sica A,Garlanda C,et al.The Yin-Yang of tumor-associated macrophages in neoplastic progression and immun1e surveillance.Immunol Rev,2008,222:155-161.
5 Zahorec R.Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill.Bratisl Lek Listy,2001,102:5-14.
6 Wang L,Liang D,Xu X,et al.The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer.Oncol Lett,2017,14:6449-6456.
7 Liu X,Qu JK,Zhang J,et al.Prognostic role of pretreatment neutrophil to lymphocyte ratio in breast cancer patients:A meta-analysis.Medicine (Baltimore),2017,96:e8101.
8 Smith RA,Bosonnet L,Raraty M,et al.Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma.Am J Surg,2009,197:466-472.
9 Zhao QT,Yuan Z,Zhang H,et al.Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers:A meta-analysis including 3,720 patients.Int J Cancer,2016,139:164-170.
10 Pecorelli S.Revised FIGO staging for carcinoma of the vulva,cervix,and endometrium.Int J Gynaecol Obstet,2009,105:103-104.
11 Bui JD,Schreiber RD.Cancer immunosurveillance,immunoediting and inflammation:independent or interdependent processes?.Curr Opin Immunol,2007,19:203-208.
12 Gregory AD,Houghton AM.Tumor-Associated Neutrophils:New Targets for Cancer Therapy.Cancer Res,2011,71:2411-2416.
13 Li MX,Liu XM,Zhang XF,et al.Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer:a systematic review and meta-analysis.Int J Cancer,2014,134:2403-2413.
14 Paramanathan A,Saxena A,Morris DL.A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours.Surg Oncol,2014,23:31-39.
15 Laohawiriyakamol S,Mahattanobon S,Laohawiriyakamol S,et al.The pre-treatment neutrophil-lymphocyte ratio:a useful tool in predicting non-sentinel lymph node metastasis in breast cancer cases.Asian Pac J Cancer Prev,2017,18:557-562.
16 Wang SC,Chou JF,Strong VE,et al.Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specificsurvival in resectable gastroesophageal junction and gastric adenocarcinoma.Ann Surg,2016,263:292-297.
17 Viers BR,Boorjian SA,Frank I,et al.Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinomaof the bladder undergoing radical cystectomy.Eur Urol,2014,66:1157-1164.
18 Sahin F,Aslan AF.Relationship between inflammatory and biological markers and lung cancer.J Clin Med,2018,7:160.
19 HanByoul C,Hye WH,Sang WK,et al.Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment.Cancer Immunol Immunother,2009,58:15-23.
20 Aoyama T,Takano M,Miyamoto M,et al.Pretreatment neutrophil-to-lymphocyte ratio was a predictor of lymph node metastasis in endometrial cancer patients.Oncol,2019,96:259-267.
21 Haruma T,Nakamura K,Nishida T,et al.Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer.Anticancer Research,2015,35:337-344.
22 Fridlender ZG,Sun J,Mishalian I,et al.Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils.PLOS One,2012,7:e31524.
23 Paramanathan A,Saxena A,Morris D.A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on longterm outcomes after curative intent resection of solid tumours.Surg Oncol,2014,23:31-39.
(收稿日期:2020-12-23)




