·实验研究·
宫颈癌(cervical cancer,CESC)属于女性生殖系统恶性肿瘤,发病率及死亡率较高,对女性生命和健康存在极大威胁。据统计,全球每年新发病例约53万人,死亡人数高达27万人[1]。临床统计资料显示,80%以上有夫妻生活经历的女性存在程度不等的宫颈疾病困扰,而处于17岁~28岁阶段的女性更易患宫颈疾病,一些女性未及30岁即发生了早期宫颈病变,有的甚至出现晚期宫颈病变,在发病年龄方面相较于以往提早了近十年[2]。在恶性肿瘤的迁移与侵袭活动中,上皮间质转化(epithelial mesenchymal transition,EMT)发挥着关键性影响。肿瘤的形成与发展过程涉及诸多基因以及步骤,其中EMT对于源于上皮的恶性肿瘤迁移与侵袭行为发挥着关键性影响[3]。EMT,即在一定生理与病理环境中上皮细胞转分化为间充质细胞的行为。此行为发生期间,上皮细胞的细胞极性、细胞间的黏附性逐渐丧失,游走性能与侵入能力显著提升[4]。叉头框C1蛋白 (forkhead box protein C1, FOXC1)是一种新型转录因子,研究显示,FOXC1在恶性肿瘤发病、进展过程中发挥着重要作用,例如,在鼻咽癌中检测到FOXC1的高表达,而FOXC1通过上调波形蛋白、纤维连接蛋白和N-cadherin的表达[5],在EMT中起到关键作用。FOXC1在乳腺癌中是EGFR功能的关键调节因子,目前已发现FOXC1的显著上调可通过激活Hedgehog信号通路来调控癌症干细胞特性的丰富[6]。在肝细胞癌中,FOXC1通过调节EMT参与微血管浸润[7]。有报道表明,FOXC1的异常表达与多种癌症的发生和发展有关,包括乳腺癌、肝细胞癌、胰腺癌和非小细胞肺癌[8-11]。然而,FOXC1的表达及其在宫颈癌中的潜在作用尚不清楚。本研究旨在探讨沉默FOXC1的宫颈癌细胞的侵袭及转移能力的影响情况,现将结果报告如下。
正常宫颈细胞NC104与人宫颈癌 CaSki、ME-180、HeLa细胞购于中科院上海肿瘤研究所,细胞均用含10%胎牛血清、100 U/mL 青霉素、100 μg/mL链霉素的DMEM培养液,置于37℃、5% CO2的恒温培养箱中培养。引物设计后由上海生工合成,LipofectamineTM转染试剂购于汉恒生物,pLKO.1-shFOXC1(本实验室克隆)。β-catenin抗体、Vimentin抗体、E-cadherin抗体购于美国Affinty 公司,TRANSWELL小室及Matrigel基质胶购于corning公司。DMEM培养基和小牛血清购自Gibco公司。
1.细胞总RNA用TRIzol试剂盒提取:RT-PCR 95℃ 30 s,95℃ 5 s 40个循环,60℃ 30 s。引物序列如下。
FOXC1:
5′-CTG CCC GAC TAC TCT CTG C-3′
5′-CAC CGA GTG GAA GTT CTG C-3′;
GAPDH:
5′-CGA GAT CCC TCC AAA ATC AA-3′
5′-TTC ACA CCC ATG ACG AAC AT-3′。
采用2-ΔΔCT法计算FOXC1 mRNA相对表达量。
2.Western Blot检测四种宫颈癌细胞中FOXC1基因的表达:FOXC1蛋白表达采用Western blot法。收集四组细胞,加入蛋白裂解液,冰上裂解充分约30 min,提取总蛋白,进行蛋白定量检测并制备蛋白样品。SDS-PAGE电泳,湿转法转膜,5% BSA摇床室温封闭1 h,1×TBST洗涤3次,每次15 min。加入FOXC1(1∶1 000)和内参GAPDH(1∶1000)一抗,4 ℃孵育过夜,1×TBST洗涤3次,每次15 min;加入二抗(1∶2 000)室温杂交1 h,1×TBST洗涤3次,每次15 min;ECL显影、曝光。实验均重复3次以上,采用Image J软件分析蛋白条带灰度值,以目的条带与内参条带灰度值的比值计算FOXC1蛋白相对表达量。根据RT-PCR及Western blot检测宫颈癌HeLa细胞中FOXC1表达量较高。
取对数生长期的HeLa细胞,无血清培养基重悬,调整细胞密度为4×105/mL,6孔板中每孔2 mL细胞悬液接种,常规培养。待细胞状态良好且融合度达60%~80%时,更换为无血清培养基。实验分为3组。 (1)对照组:转染阴性的Hela细胞; (2)阴性对照组:用pc DNA3.1 (+) -neo质粒转染Hela细胞; (3) 转染特异性siRNA组:用pLKO.1-shFOXC1转染Hela细胞。转染方法参照Lipofectamine2000转染方法进行操作。转染48 h后进行培养, 3 d后嘌呤霉素筛选阳性细胞连代培养,通过倒置显微镜观察转染效率,RT-PCR及Western Blot检测转染后细胞中FOXC1mRNA及蛋白表达,达到稳定转染后进行后续实验。
1.检测转染后细胞迁移能力:制备细胞悬液,调整细胞浓度为5×105/mL。取细胞悬液200 μL加入Transwell上室, 下室中加入500 μL的DMEM培养液,小心消除基质胶与培养液间的气泡。48 h后进行取样,吸净chamber上室内液体,无菌镊子取出chamber,移至800 μL甲醇溶液内,室温固定30 min。从甲醇溶液中取出chamber,吸干甲醇溶液,移至800 μL Giemsa染色液中,室温染色30 min,后用去离子水冲洗后吸干液体,湿棉棒擦去chamber上室未迁移细胞,PBS冲洗3遍。倒置显微镜下随机取9视野,并记数。
2.检测转染后细胞侵袭能力:用Matrigel基质胶包被Transwell小室基底膜,Matrigel胶4℃过夜融化备用,用4℃预冷的无血清DMEM培养基1∶3稀释Matrigel至终浓度为1 mg/mL,冰上操作;灭菌枪头包被小室的底部,37℃静置1 h,使其干成胶状。其余方法同迁移实验,并记数。
收集三组转染48 h的细胞,实验分组包括空白对照组(HeLa)、空载组(GFP- HeLa)、HeLa-FOXC1(-)组,分别提取细胞总蛋白,Western blot检测 β-catenin、E-cadherin及Vimentin蛋白的表达差异,参照二-2中采用Western blot法检测β-catenin、Vimentin及E-cadherin蛋白相对表达量。
应用PCR及Western Blot方法检测正常宫颈细胞NC104及宫颈癌 CaSki、ME-180、HeLa细胞株中FOXC1表达。PCR及Western Blot结果提示宫颈癌HeLa细胞中FOXC1高表达。
1.以MOI=30 慢病毒感染HeLa细胞系,48 h后荧光显微镜观察GFP荧光蛋白的表达情况见图2。并以嘌呤霉素筛选出稳定表达FOXC1 shRNA的细胞,流式细胞仪分选计数,其平均荧光细胞比率为(93.71±0.26)%。
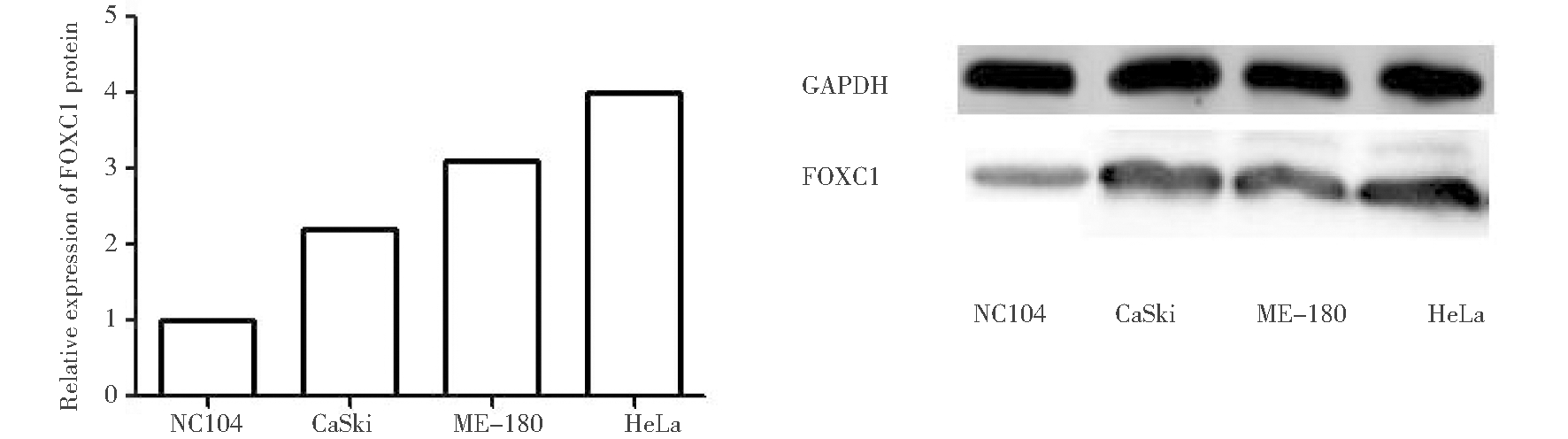
图1 RT-PCR检测四组细胞中FOXC1 mRNA相对表达量及Western Blot检测四组细胞中FOXC1表达情况
Figure 1 The relative expression of FOXC1 mRNA detected by RT-PCR
and the expression of FOXC1 protein detected by Western blot
2.转染48 h后,PCR检测FOXC1mRNA的表达情况:各种的相对表达量见表1,其中转染FOXC1特异性shRNA组与阴性对照组、对照组相比较,FOXC1的mRNA相对表达量显著降低,差异具有统计学意义。
表1 FOXC1基因在各组中的表达情况![]()
Table 1 The expression of FOXC1 gene
in three groups of ![]()

GroupRelative content of foxc1 mRNASh FOXC1 group0.1302±0.0398∗▲Negative control group1.0034±0.1450Control group 1.0106±0.0230
compared with the negative control group,*P<0.01;compared with the control group,▲P<0.01
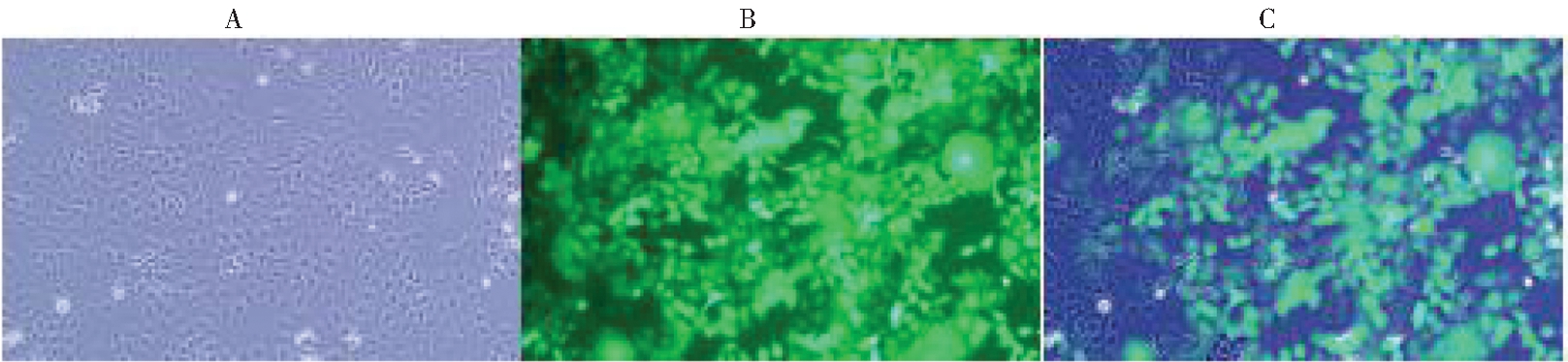
A: Observation results of ordinary light microscope; B: Observation results of green fluorescence microscope;C: Fusion diagram of A and B
图2 荧光显微镜下观察HeLa-FOXC1(-)细胞系(放大倍数,×10倍)的荧光显示转染情况
Figure 2 The transfection of -FOXC1 (-) cell line
was observed under fluorescence microscope (magnification, ×10)
3.转染48 h后Western Blot检测转染后三组细胞中FOXC1蛋白水平表达:转染48 h后分别提取感染阴性组细胞HeLa、感染空载体组细胞HeLa-GFP以及感染FOXC1 shRNA组细胞HeLaFOXC1(-)的总蛋白,通过Western blot方法检测三种细胞中FOXC1蛋白的表达差异,GAPDH作为内参,结果可见HeLa-FOXC1(-)组细胞内FOXC1蛋白的表达明显降低(P<0.01)。见图3。

图3 三组细胞中FOXC1蛋白相对表达情况
Figure 3 The relative expression of FOXC1 protein in three groups
1.使用Transwell小室24 h后检测HeLa细胞系,GFP稳定株和FOXC1的干扰稳定株的细胞迁移情况:迁移结果显示,敲减FOXC1基因后三组细胞迁移能力比较, HeLa-FOXC1(-)组细胞迁移能力明显下降(P<0.05),HeLa组与 HeLa-GFP组无明显差异。见图4。
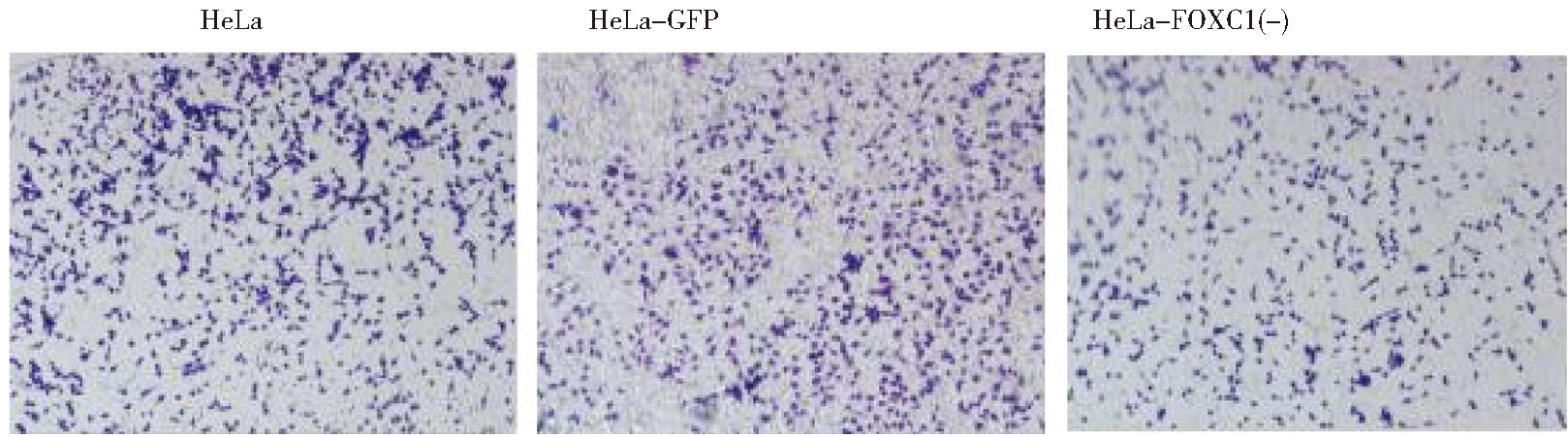
图4 Transwell小室24 h后三组细胞迁移情况(×40)
Figure 4 Cell migration of crystal violet staining of shFOXC1 and control group cells that
crossed the polycarbonate membrane of the Transwell chamber (×40)
2.用Transwell小室24 h时分别检测HeLa细胞系,GFP稳定株和FOXC1的干扰稳定株的细胞侵袭情况:Transwell侵袭实验结果显示,敲减FOXC1基因后三组细胞侵袭能力比较, HeLa-FOXC1(-)组细胞侵袭能力明显下降(P<0.05),HeLa组与 HeLa-GFP组无明显差异。见图5。
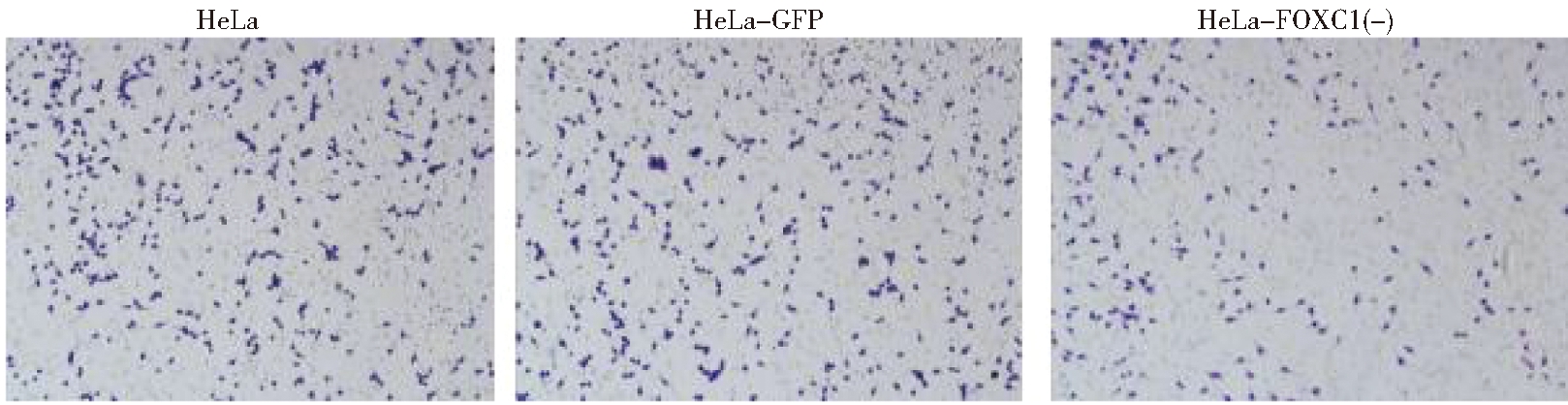
图5 Transwell小室24 h后三组细胞侵袭情况(×40)
Figure 5 Cell invasion of crystal violet staining of the shFOXC1 and control group
cells that crossed the Matrigel-coated polycarbonate membrane of the Transwell chamber to (×40)
由图6所示,A:使用WB法检测HeLa细胞系,GFP稳定株和FOXC1的干扰稳定株中E-cadherin,β-catenin和vimentin的蛋白表达情况;B:使用WB法检测HeLa细胞系,GFP稳定株和FOXC1的干扰稳定株中E-cadherin的蛋白表达情况统计结果(P<0.05);C:使用WB法检测HeLa细胞系,GFP稳定株和FOXC1的干扰稳定株中β-catenin的蛋白表达情况统计结果(P<0.05);D:使用WB法检测HeLa细胞系,GFP稳定株和FOXC1的干扰稳定株中vimentin的蛋白表达情况统计结果(P<0.05)。结果可见,HeLa-FOXC1(-)组细胞内β-catenin及Vimentin蛋白的表达明显降低,E-cadherin蛋白的表达有增高。
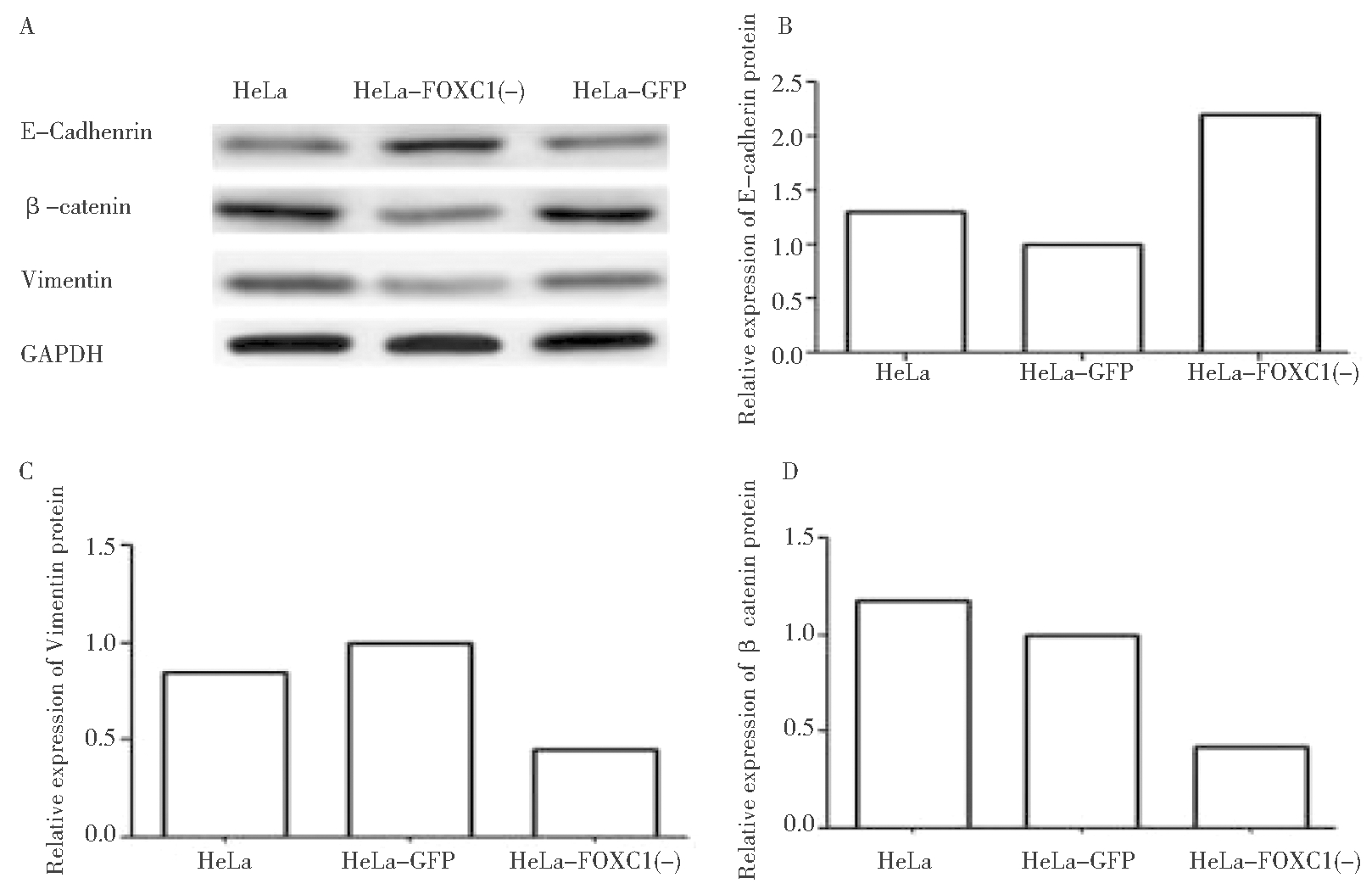
图6 Western blot检测感染前后各组细胞中 β-catenin、E-cadherin
及Vimentin蛋白的表达情况及各组细胞中蛋白的相对表达量
Figure 6 The expression of protein and the relative expression
quantity ofβ-catenin, E-Cadhenrin and Vimentin detected by Western blot
侵袭和转移是肿瘤临床治疗的难题及肿瘤患者死亡的主要原因。恶性肿瘤中上皮-间质转变现象的发生是引起肿瘤远处转移的关键步骤。EMT出现的分子标志为N-cadherin、Fibronectin与Vimentin这3类间质标志物表达的提升,β-Catenin与E-cadherin这2类上皮标志物表达的缺失或降低[12-14]。本课题组前期研究中发现,FOXC1基因在宫颈癌患者的临床病理中高表达,且随着期别进展,FOXC1基因的表达有明显增高,差异有统计学意义。本研究主要通过慢病毒RNA干扰技术,敲减宫颈癌HeLa细胞中FOXC1基因的表达,并通过倒置荧光显微镜及PCR、Western Blot方法验证在HeLa-FOXC1(-)细胞中,FOXC1基因的mRNA及蛋白水平明显降低,通过细胞培养技术获得稳定沉默FOXC1的细胞系,通过Transwell迁移及侵袭实验验证,感染FOXC1-shRNA组细胞的迁移及侵袭能力明显降低,进一步通过Western blot检测三组细胞中β-catenin、E-cadherin及Vimentin的表达,发现FOXC1敲除组细胞系内β-catenin及Vimentin蛋白的表达明显降低,E-cadherin蛋白的表达有增高,进一步验证敲除FOXC1基因的宫颈癌细胞后,下调上皮-间转化(EMT)过程中与转移有关蛋白(β-catenin、Vimentin)的表达,并增加钙黏蛋白(E-cadherin)的表达,使其侵袭及转移能力随之下降。由此可见,FOXC1和EMT在宫颈癌发展过程中有一定作用,其异常表达可能作为宫颈癌检查新的标志物。
宫颈癌是女性非常常见的妇科癌症之一,现全世界范围内每年仍有超过30万人因此病死亡[2],当前形势下其具体病因及发病机制仍不明确。临床上,宫颈癌的进展是从局部浸润、侵犯周围器官及淋巴结转移与远处转移,其特点是早期可发生淋巴结及远处转移。据报道,无淋巴转移的宫颈癌患者5年生存率为82.3%,有淋巴转移的5年生存率为50.8%[15]。因此,控制肿瘤的侵袭转移是提高宫颈癌患者生存率的主要方法之一[16-17]。肿瘤的基因靶向治疗是未来癌症治疗的主要手段,在不久的将来,相信FOXC1有望成为宫颈癌治疗的新靶点。
1 Small W,Bacon MA,Bajaj A,et al.Cervicalcancer:A global health crisis.Cancer,2017,123:2404-2412.
2 Duenas-Gonzalez A,Campbell S.Global strategies for the treatment of early-stage and advancedcervical cancer.Curr Opin Obstet Gynecol,2016,28:11-17.
3 Thompson JC,Hwang WT,Davis C,et al.Gene signatures of tumor inflammation and epithelial-to-mesenchymal transition (EMT) predict responses to immune checkpoint blockade in lung cancer with high accuracy.Lung cancer,2020,1:131-139.
4 Li Yl,Wang T,Sun Y,et al.p53-Mediated PI3K/AKT/mTOR Pathway Played a Role in PtoxDpt-Induced EMT Inhibition in Liver Cancer Cell Lines.Oxid Med Cell Longev,2019,11:2531-2538.
5 Ou-Yang L,Xiao SJ,Liu P,et al.Forkhead box C1 induces epithelialmesenchymal transition and is a potential therapeutic target in nasopharyngeal carcinoma.Mol Med Rep,2015,12:8003-8009.
6 Han B,Qu Y,Jin Y,et al.FOXC1 activates smoothened-independent hedgehog signaling in basal-like breast cancer.Cell Rep,2015,13:1046-1058.
7 Xu ZY,Ding SM,Zhou L,et al.FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition.Int J Biol Sci,2012,8:1130-1141.
8 Xia L,Huang W,Tian D,et al.Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepa-tocellular carcinoma.Hepatology,2013,57:610-624.
9 Wang Z,Qu L,Deng B,et al.STYK1 promotes epithelial-mesenchymal transition and tumor metastasis in human hepatocellular carcinoma through MEK/ERK and PI3K/AKT signaling.Sci Rep,2016,6:33205.
10 Song Y,Washington MK,Crawford HC.Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal transition in pancre-atic cancer.Cancer Res,2010,70:2115-2125.
11 Ray PS,Wang J,Qu Y,et al.FOXC1 is a potential prog-nostic biomarker with functional significance in basal-like breast cancer.Cancer Res,2010,70:3870-3876.
12 Schmalhofer O,Brabletz S,Brabletz T.E-cadherin,beta-catenin,and ZEB1 in malignant progression of cancer.Cancer Metastasis Rev,2009,28:151-166.
13 Kowalski PJ,Rubin MA,Kleer CG.E-cadherin expression in primary carcinomas of the breast and its distant metastases.Breast Cancer Res,2003,5:217-222.
14 Lamouille S,Xu J,Derynck R.Molecular mechanisms of epithelial-mesenchymal transition.Nat Rev Mol Cell Biol,2014,15:178-196.
15 Li D,Cai J,Kuang Y,et al.Surgical-pathologic risk factors of pelvic lymph node metastasis in stage Ib1-IIb cervical cancer.Acta Obstet Gynecol Scand,2012,91:802-809.
16 KrillL S,Tewari KS.Exploring the Therapeutic Rationale for Angiogenesis Blockade in Cervical Cancer.Clin Ther,2015,37:9-19.
17 Tewari KS,Monk BJ.New strategies in advanced cervicalcancer:from angiogenesis blockade to immunotherapy.Clin Cancer Res,2014,20:5349-5358.