隐睾症是常见的先天性泌尿生殖系统畸形,在足月男婴中的发病率高达3%~4%[1]。隐睾已成为男性不育的重要原因。早期睾丸固定术或激素治疗能使隐睾睾丸降至阴囊内,但不能有效恢复患者的生精功能[2-3]。视黄酸(retinoic acid, RA)是维生素A在体内氧化代谢的一类具有生物活性的产物,具有维持雄性生殖系统发育和正常的作用[4]。研究证实,RA及其受体是精子发生的重要因素,缺乏维生素A的动物精子生成过程停止,大多数生殖细胞被降解,但RA治疗可恢复生精过程[5]。近期研究证实,微小RNA(micro RNAs, miRNAs)在男性不育等相关疾病中发挥重要调控作用。例如,miR-4485-3p在特发性弱精子症男性精子中低表达,并可能是线粒体功能障碍与弱精子症的关键分子[6]。MiR-509-5p与男性不育呈负相关,在伴有成熟停滞的不育男性中低表达,可抑制睾丸生殖细胞肿瘤细胞体外增殖,并促进凋亡[7]。此外,有研究证实,RA可通过调控miRNA的表达影响疾病的发展进程[8-9]。本研究通过构建氟他胺致隐睾小鼠模型,探讨RA对隐睾生精细胞增殖分化及对隐睾组织中miR-210表达的影响及机制。
材料与方法
一、主要试剂
氟他胺及RA购于美国Sigma公司。一步法逆转录试剂盒购于日本TaKaRa公司。RT-qPCR引物购于北京金唯智生物技术有限公司。Trizol试剂和RIPA裂解液购于南京KeyGEN公司。C-kit和PLZF一抗、HRP标记的二抗购于美国Abcam公司。
二、研究方法
1.隐睾小鼠模型构建及分组:所有BALB/c小鼠适应性喂养后将雌雄小鼠按2:1比例合笼,随机分为:正常对照组(Control组)、氟他胺组(Flu组)、视黄酸治疗组(RA组)、Flu+miR-210敲降组、Flu+miR-210过表达组及Flu+miR-210过表达+RA组,每组各10只。合笼后检测阴栓,以观察到阴栓之日为妊娠0 d。从妊娠后12~21 d每天给孕鼠灌胃,700 mg/kg。判断标准:幼鼠出生后4周观察幼鼠有无阴囊或睾丸是否下降至阴囊。在氟他胺组基础上,将antagomiR-210和agomiR-210通过尾静脉分别注射至幼鼠体内,8 mg/kg。
2. RT-qPCR实验检测miR-210、C-kit和PLZF mRNA的表达水平:使用Trizol试剂提取各组小鼠睾丸组织中的总RNA,使用一步法逆转录试剂盒将全部RNA逆转录成cDNA,随后进行RT-qPCR检测miR-210、C-kit和PLZF mRNA的表达水平。反应程序为:预变性95℃ 30 s、95℃ 5 s、60℃ 30 s、45个循环。以U6和GAPDH为内参,采用2-△△Ct法定量。
3. Western blot检测C-kit和PLZF蛋白的表达水平:使用RIPA裂解液提取各组小鼠睾丸组织总蛋白,BCA法测定蛋白浓度。以30 μg/孔上样量行10% SDS-PAGE电泳,半干法转移至PVDF膜上,5%脱脂奶粉室温封闭2 h,加入1∶2 000稀释好一抗,4℃过夜孵育;次日,弃一抗,加入1∶3 000稀释的HRP标记的二抗,室温孵育2 h;加入ECL化学发光液进行显影,最后在凝胶成像仪上进行曝光拍照,Image J分析蛋白条带灰度值。
4.统计学分析:用SPSS 20.0软件进行统计学分析,数据以![]() 表示,两组间比较采用t检验,多组间比较采用单因素方差分析,以P<0.05表示差异有统计学意义。
表示,两组间比较采用t检验,多组间比较采用单因素方差分析,以P<0.05表示差异有统计学意义。
结 果
一、RA促进隐睾生精细胞增殖分化
RT-qPCR和Western blot结果显示,与Control组相比,Flu组睾丸组织中C-kit和PLZF mRNA和蛋白表达水平均显著下调,RA治疗后C-kit和PLZF mRNA和蛋白表达水平明显恢复(图1A和图1B)。该结果说明,RA可促进隐睾生精细胞增殖分化。
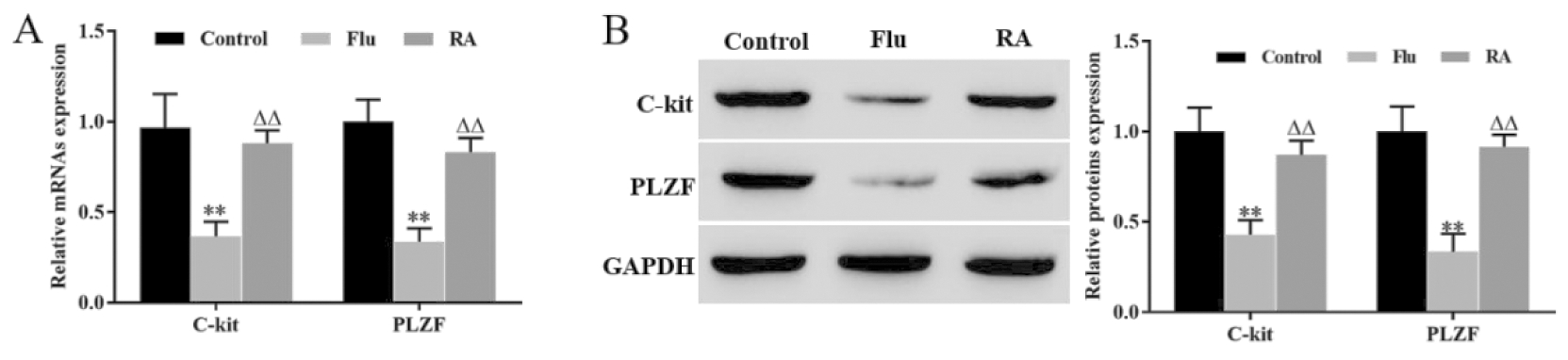
**P<0.01, vs Control group; △△P<0.01, vs Flu group
图1 RA促进隐睾生精细胞增殖分化
Figure 1 RA promoted the proliferation and differentiation of cryptorchidogenic spermatocytes
A. The expression levels of c-kit and PLZF mRNA were detected by RT qPCR; B. The expression levels of c-kit and PLZF proteins were detected by Western blot
二、RA下调隐睾睾丸组织中miR-210的表达
RT-qPCR结果显示,与Control组相比,Flu组睾丸组织中miR-210表达水平显著上调,而RA治疗可回复氟他胺对miR-210的上调作用(图2)。该结果说明,RA可下调隐睾睾丸组织中miR-210的表达水平。
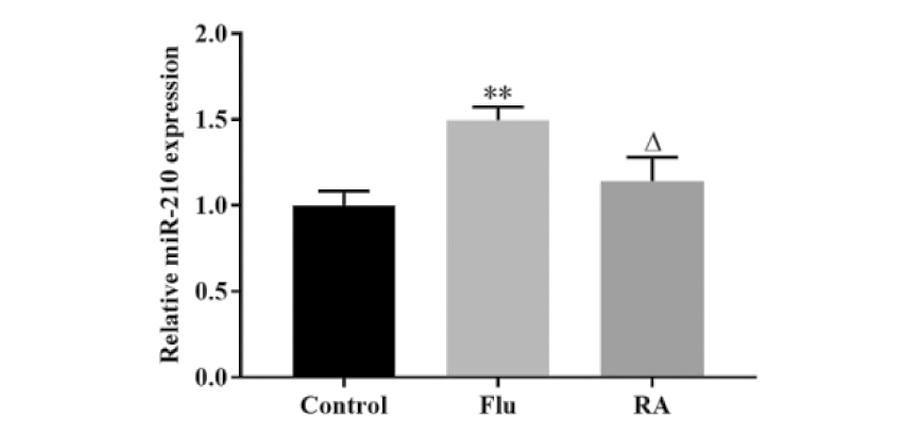
**P<0.01, vs Control group; △P<0.05, vs Flu group
图2 RA下调隐睾睾丸组织中miR-210的表达
Figure 2 RA downregulated the expression of miR-210 in cryptorchidical testicular tissue
三、敲降miR-210促进隐睾生精细胞增殖分化
RT-qPCR结果显示,与Flu组相比,敲降miR-210显著下调隐睾睾丸组织中miR-210的表达水平(P<0.05,图3A)。RT-qPCR和Western blot结果显示,与Flu组相比较,敲降miR-210组显著上调睾丸组织中C-kit和PLZF mRNA和蛋白表达水平(P<0.05,图3B和图3C)。该结果说明,敲降miR-210可促进隐睾生精细胞增殖分化。
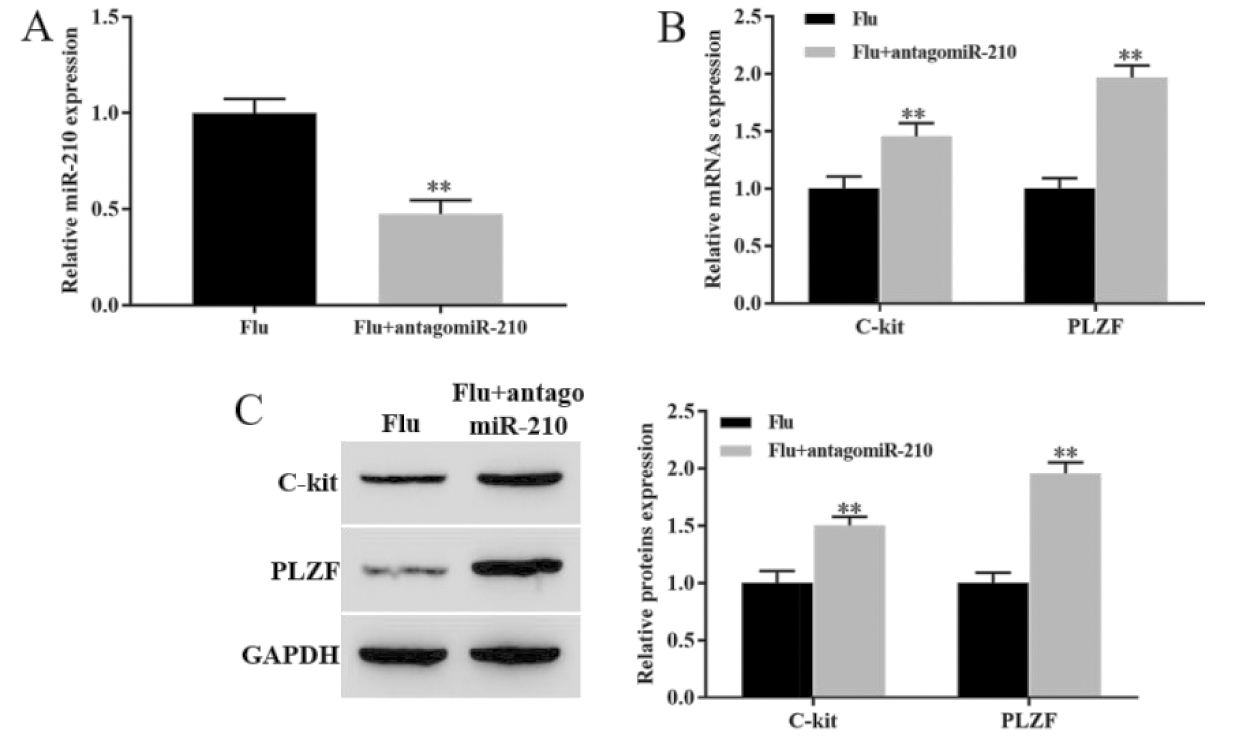
**P<0.01, vs Flu group
图3 敲降miR-210促进隐睾生精细胞增殖分化
Figure 3 Knockdown of miR-210 promoted the proliferation and differentiation of cryptorchidogenic spermatocytes
A. The expression level of mir-210 was detected by RT qPCR by RT qPCR;B. The expression levels of c-kit mRNA and PLZF mRNA were detected by RT qPCR;C. The expression levels of c-kit and PLZF proteins were detected by Western blot
四、RA下调miR-210促进隐睾生精细胞增殖分化
RT-qPCR结果显示,与Flu组相比,过表达miR-210组显著上调睾丸组织中miR-210的表达水平,而过表达miR-210同时用RA治疗,可回复miR-210的表达水平(图4A)。RT-qPCR和Western blot结果显示,miR-210过表达组睾丸组织中C-kit和PLZF mRNA和蛋白表达水平均显著低于Flu组(P<0.05),而RA治疗可回复睾丸组织中C-kit和PLZF mRNA和蛋白表达水平,即RA治疗可回复过表达miR-210对隐睾生精细胞增殖分化的抑制作用(图4B和C)。该结果说明,RA可下调miR-210促进隐睾生精细胞增殖分化。
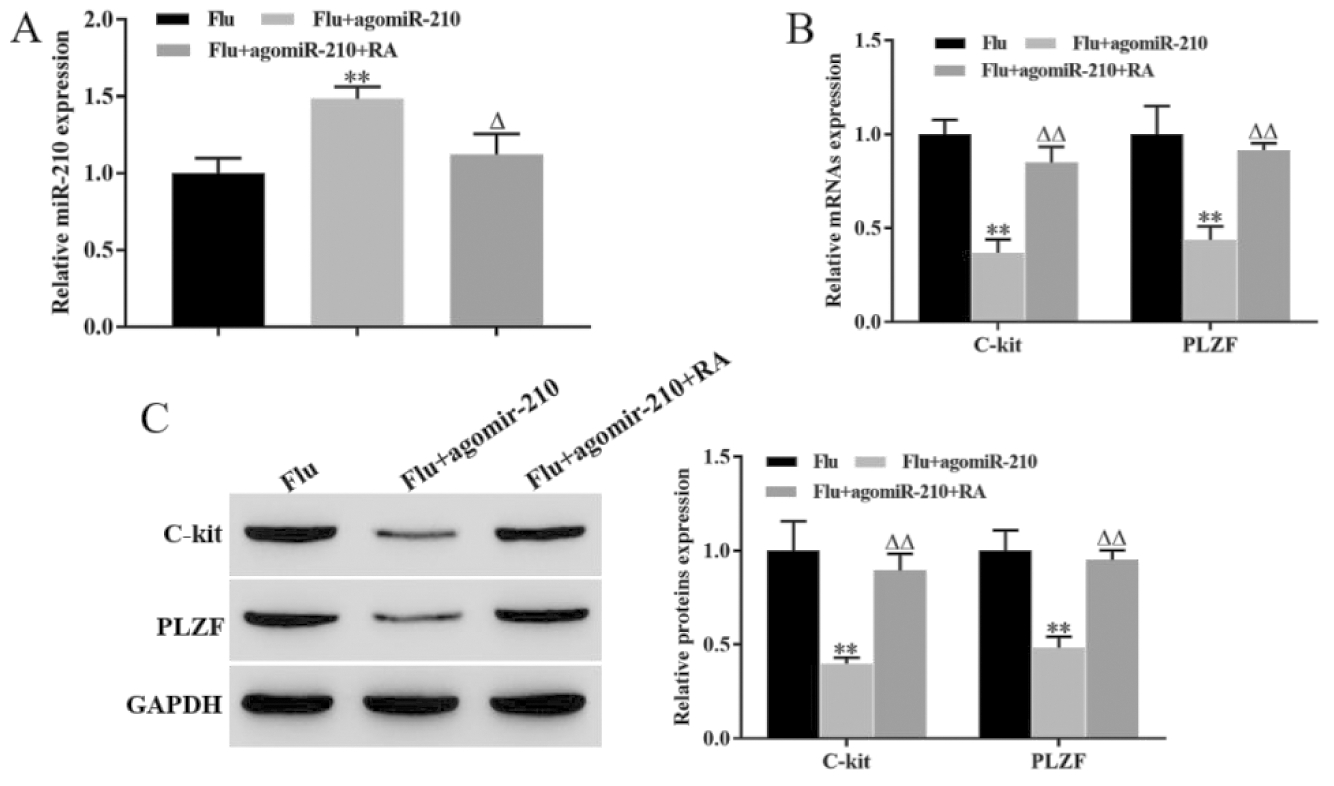
**P<0.01, vs Flu group; △P<0.05, △△P<0.01, vs Flu + agomiR-210 group
图4 RA下调miR-210促进隐睾生精细胞增殖分化
Figure 4 RA promoted the proliferation and differentiation of cryptorchidogenic spermatocytes by downregulating miR-210
A. The expression level of mir-210 was detected by RT qPCR by RT qPCR; B. The expression levels of c-kit mRNA and PLZF mRNA were detected by RT qPCR; C. The expression levels of c-kit and PLZF proteins were detected by Western blot
讨 论
精原干细胞的分裂呈现为边复制边分化的不对称性,这是雄性哺乳动物能够连续不断进行生精过程的基础[10]。而 PLZF和C-kit是精原干细胞增殖分化的关键基因[11-12]。研究表明,PLZF参与精原干细胞的自我复制过程,是维持精原干细胞数目不可或缺的因子[13]。本研究发现,隐睾睾丸组织(氟他胺组)中PLZF mRNA和蛋白的表达水平均明显下降,表明隐睾小鼠精原细胞的自我增殖发生异常,精原细胞数量减少。而使用RA治疗后,RA组中PLZF mRNA和蛋白的表达水平较氟他胺组显著上调,这说明RA可促进精原细胞的自我增殖,维持精原细胞数量。C-kit即跨膜酪氨酸激酶受体,在精原细胞分化过程中起着重要作用[14]。近期研究表明,C-kit通过与其配体干细胞因子(stem cell factor,SCF)结合,可促使精原细胞进行DNA合成,在一定程度上反应了精原细胞分化活动的强弱,是精原细胞分化的标志之一[15]。本研究发现,C-kit在隐睾睾丸组织中表达水平显著下调,RA治疗明显恢复其表达水平。这反映出隐睾精原细胞分化出现障碍,而RA能上调C-kit的表达,促进精原细胞分化。
MiRNAs是一类长约22 nt的单链非编码小RNA,在机体生理和病理过程发挥重要调控作用[16]。研究表明,miRNAs在包括隐睾在内的多种男性不育疾病中异常表达[17]。例如,miR-34c在隐睾患者睾丸组织和隐睾小鼠模型中均显著下调,其异常表达破坏了精原干细胞的自我更新和分化平衡,最终破坏了隐睾精子的产生[18]。Moritoki等人[19]研究证实,miR-135a-5p在隐睾中低表达,并通过与叉头框蛋白O1重组蛋白(Forkhead box transcription factor O1,FOXO1)相互作用参与精原干细胞的维持。本研究发现,miR-210在隐睾睾丸组织中高表达,而RA治疗可下调其表达水平。在隐睾小鼠体内稳定表达miR-210拮抗剂antagomiR-210后,PLZF和C-kit mRNA和蛋白的表达水平均显著上调,稳定表达agomiR-210后,PLZF和C-kit mRNA和蛋白的表达水平均明显低于氟他胺组,而稳定表达agomiR-210后,再进行RA治疗则可恢复PLZF和C-kit的表达水平。这说明,高水平的miR-210能引起精原细胞增殖和分化异常,而RA治疗可下调隐睾睾丸组织中miR-210的表达,促进PLZF和C-kit的表达。
综上所述,miR-210在隐睾睾丸组织中高表达,RA可下调miR-210的表达,并通过miR-210促进隐睾生精细胞的增殖和分化。
1 Lee PA,Houk CP.Cryptorchidism.Curr Opin Endocrinol Diabetes Obes,2013,20:210-216.
2 Yu CJ,Long CL,Wei Y,et al.Evaluation of Fowler-Stephens orchiopexy for high-level intra-abdominal cryptorchidism:A systematic review and meta-analysis.Int J Surg,2018,60:74-87.
3 Chua ME,Mendoza JS,Gaston MJV,et al.Hormonal therapy using gonadotropin releasing hormone for improvement of fertility index among children with cryptorchidism:A meta-analysis and systematic review.J Pediatr Surg,2014,49:1659-1667.
4 Balmer JE,Blomhoff R.Gene expression regulation by retinoic acid.J Lipid Res,2002,43:1773-1808.
5 Li X,Long XY,Xie YJ,et al.The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders.Clin Chim Acta,2019,497:54-60.
6 Heidary Z,Zaki-Dizaji M,Saliminejad K,et al.MiR-4485-3p expression reduced in spermatozoa of men with idiopathic asthenozoospermia.Andrologia,2020,52:e13539.
7 Sun JX,Niu L,Gao SX,et al.MiR-509-5p downregulation is associated with male infertility and acts as a suppressor in testicular germ cell tumor cells through targeting MDM2.Onco Targets Ther,2019,12:10515-10522.
8 Perri M,Caroleo MC,Liu NN,et al.9-cis Retinoic acid modulates myotrophin expression and its miR in physiological and pathophysiological cell models.Exp Cell Res,2017,354:25-30.
9 Wu HB,Zhao JM,Fu BB,et al.Retinoic acid-induced upregulation of miR-219 promotes the differentiation of embryonic stem cells into neural cells.Cell Death Dis,2017,8:e2953.
10 Hermann BP,Chen KR,Singh A,et al.The mammalian spermatogenesis single-cell transcriptome,from spermatogonial stem cells to spermatids.Cell Rep,2018,25:1650-1667. e1658.
11 Sharma M,Srivastava A,Fairfield HE,et al.Identification of EOMES-expressing spermatogonial stem cells and their regulation by PLZF.Elife,2019,8.
12 Tang RL,Fan LQ.PLZF(pos)c-KIT(pos)-delineated A1-A4-differentiating spermatogonia by subset and stage detection upon Bouin fixation.Asian J Androl,2019,21:309-318.
13 Wang JJ,Li JM,Xu W,et al.Androgen promotes differentiation of PLZF(+) spermatogonia pool via indirect regulatory pattern.Cell Commun Signal,2019,17:57.
14 Lee R,Lee WY,Park HJ,et al.Stage-specific expression of DDX4 and c-kit at different developmental stages of the porcine testis.Anim Reprod Sci,2018,190:18-26.
15 Unni S K,Modi DN,Pathak SG,et al.Stage-specific localization and expression of c-kit in the adult human testis.J Histochem Cytochem,2009,57:861-869.
16 Lu TX,Rothenberg ME.MicroRNA.J Allergy Clin Immunol,2018,141:1202-1207.
17 Yadav SK,Pandey A,Kumar L,et al.The thermo-sensitive gene expression signatures of spermatogenesis.Reprod Biol Endocrinol,2018,16:56; DOI:10. 1186/s12958-018-0372-8.
18 Huang ZY,Tang DD,Gao JJ,et al.miR-34c disrupts spermatogonial stem cell homeostasis in cryptorchid testes by targeting Nanos2. Reprod Biol Endocrinol,2018,16:97.
19 Moritoki Y,Hayashi Y,Mizuno K,et al.Expression profiling of microRNA in cryptorchid testes:miR-135a contributes to the maintenance of spermatogonial stem cells by regulating FoxO1.J Urol,2014,191:1174-1180.