子痫前期是妊娠期并发症中常见的疾病之一,同时也是造成孕产妇及围产儿死亡的高危因素之一[1]。目前子痫前期发病机制的研究较为广泛,但确切病因仍是众说纷纭,其中滋养细胞侵入不足以及胎盘螺旋动脉重铸不足这两个原因是目前普遍公认的重要病理基础,但病因仍尚未完全明确[2-3]。长链非编码RNA缺氧诱导因子-1α-反义链1(long non-coding RNA hypoxia-inducible factor 1 alpha antisense RNA 1,LncRNA HIF1A-AS1)是一种特殊类型的自然反义RNA,其正义RNA为缺氧诱导因子-1α(hypoxia-inducible factor 1 alpha,HIF1A)mRNA[4]。自然反义RNA在结构上与其正义RNA存在互补序列,且能通过基因重组、转录碰撞等多种调控机制影响正义RNA的表达[5]。HIF1A能介导低氧反应,与血管内皮损伤等有重要联系[6]。然而有关LncRNA HIF1A-AS1、HIF1A是否与胎盘螺旋动脉重铸不足及滋养细胞侵入不足有关目前尚未可知。因此本研究着重探讨LncRNA HIF1A-AS1、HIF1A与重度子痫前期胎盘螺旋动脉重铸的相关性。
资料与方法
一、一般资料
回顾性分析2019年10月—2021年4月于本院住院分娩的92例晚发型重度子痫前期孕妇(子痫前期组)的病例资料,孕妇年龄23~32岁,平均(27.9±3.1)岁,孕周(38.5±0.5)周;选取同期86例正常妊娠孕妇作为正常妊娠组,孕妇年龄23~31岁,平均(27.3±2.8)岁,孕周(38.4±0.7)周。
纳入标准:(1)重度子痫前期孕妇符合第九版《妇产科学》子痫前期诊断和分度标准[7];(2)孕妇均为汉族,且均为单胎头位妊娠;(3)年龄>18岁,临床资料齐全;(4)既往无严重急慢性疾病。排除标准:(1)妊娠期间孕妇伴有其他妊娠合并症;(2)妊娠前期有用药史,妊娠期间存在饮酒及吸烟史;(3)合并自身免疫系统疾病、糖尿病、慢性肾炎等疾病;(4)有其他传染病史。
收集整理孕妇一般资料,主要包括年龄、分娩前体质量指数(body mass index,BMI)、孕周、孕次、产次、收缩压、舒张压、新生儿体重。研究经本院临床研究伦理委员会批准授权,符合伦理学标准,受试孕妇均签署知情同意书。
二、方法
1.主要试剂与仪器:RNA提取试剂盒(货号:K1101)购自上海瓦兰生物科技有限公司;qPCR SYBR© Green Mix(货号:KL2091aP)购自上海康朗生物科技有限公司;反转录试剂盒(货号:205311)购自德国QIAGEN公司。qRT-PCR仪(型号:CFX384)购自美国Bio-Rad公司;扫描电子显微镜(型号:JCM-7000)购自日本电子株式会社。
2.研究方法:
(1)胎盘组织中LncRNA HIF1A-AS1、HIF1A mRNA表达水平测定。胎盘娩出后,立即采集新鲜胎盘,无菌条件下在胎盘母体面4个象限中心各取1块大小为1 cm×1 cm×1 cm的胎盘组织,PBS缓冲液反复冲洗2~3次后,采用灭酶锡箔纸包好放入经焦炭酸二乙酯处理的冷冻管中保存待测。采用实时荧光定量PCR(quantitative real-time PCR,qRT-PCR)法检测胎盘组织中LncRNA HIF1A-AS1、HIF1A mRNA表达水平:采用试剂盒提取胎盘组织总RNA并进行逆转录,然后进行qRT-PCR反应,所用引物由上海生工生物工程有限公司合成(引物序列见表1)。反应条件:95 ℃预变性5 min,1个循环;95 ℃变性30 s,58 ℃ 30 s,72 ℃ 30 s,35个循环。反应体系:cDNA(50 ng/μL)2 μL,qPCR SYBR© Green Mix 10 μL,上下游引物(10 μmol/L)各0.8 μL,ddH2O 6.4 μL。每份样品均设3个重复孔,GAPDH作为内参,采用2-△△CT法计算胎盘组织中LncRNA HIF1A-AS1、HIF1A mRNA的相对表达量。△△CT=[(待测样本目的基因CT值-待测样本内参基因CT值)-(对照样本目的基因CT值-对照样本内参基因CT值)]。
表1 qRT-PCR引物序列
Table 1 qRT-PCR Primer Sequence

GeneForward primer5′-3′Reverse primer5′-3′LncRNA HIF1A-AS1CTGTCGACGCTGACTGAGTGGATACACGCTAGTGTGGAGGTGHIF1ATCGCTACTGCTGCTCGTACGTACGGATAGTAGGTGCCGTACGGAPDHGCGATGTCGTACGATAGGACCGCTAGTAGTCGATACGGCAGT
(2)胎盘螺旋动脉管壁厚度和管腔面积测定。将剥离的新鲜胎盘取组织大小为0.5 cm×0.5 cm×0.2 cm,采用PBS缓冲液冲洗后,锇酸染色、浸泡、脱水后镀金染色。于扫描电子显微镜下随机选取组织标本大于5条螺旋动脉的管腔面积和管壁厚度,计算均值做为最终所得值。
3.统计学分析:采用SPSS 25.0软件分析数据,计量资料经正态性检验符合正态分布,均以![]() 表示,两组间比较采用t检验;采用Pearson法分析晚发型重度子痫前期孕妇胎盘组织LncRNA HIF1A-AS1、HIF1A mRNA水平与各参数的相关性。P<0.05表示差异有统计学意义。
表示,两组间比较采用t检验;采用Pearson法分析晚发型重度子痫前期孕妇胎盘组织LncRNA HIF1A-AS1、HIF1A mRNA水平与各参数的相关性。P<0.05表示差异有统计学意义。
结 果
一、正常妊娠组、子痫前期组一般情况比较
正常妊娠组、子痫前期组年龄、分娩前BMI、孕周、孕次、产次、新生儿体重比较,差异无统计学意义(P>0.05);与正常妊娠组相比,子痫前期组收缩压、舒张压明显升高,差异有统计学意义(P<0.05)。见表2。
表2 正常妊娠组、子痫前期组一般情况比较![]()
Table 2 Comparison ofbasic characteristics between normal pregnancy group and preeclampsia group![]()

GroupNAgeBMI before delivery(kg/m2)Gestational week(weeks)Number of pregnancies (times)Delivery times (times)Systolic pressure(mm Hg)Diastolic pressure(mm Hg)Newborn weight(g)Normal pregnancy8627.3±2.828.1±3.138.4±0.72.2±0.71.3±0.4118.9±8.874.9±7.13 041.6±332.2Preeclampsia9227.9±3.128.7±3.138.5±0.52.3±0.41.3±0.4158.2±12.1∗106.5±8.8∗2 985.6±369.5
Note:Compared with the normal pregnancy group,*P<0.05
二、正常妊娠组、子痫前期组胎盘螺旋动脉管壁厚度及管腔面积比较
与正常妊娠组相比,子痫前期组平均管壁厚度明显升高,平均管腔面积明显降低,差异有统计学意义(P<0.05)。见表3。
表3 正常妊娠组、子痫前期组胎盘螺旋动脉管壁厚度及管腔面积比较![]()
Table 3 Comparison of wall thickness and lumen area of placental spiral artery between normal pregnancy group ![]()

GroupNAverage pipe wall thickness(μm)Average lumen area(μm2)Normal pregnancy8699.5±7.3187.7±24.5Preeclampsia92120.8±8.9∗135.6±25.2∗
Note:Compared with the normal pregnancy group,*P<0.05
三、正常妊娠组、子痫前期组胎盘组织LncRNA HIF1A-AS1、HIF1A mRNA水平比较
与正常妊娠组相比,子痫前期组胎盘组织HIF1A mRNA水平明显升高,LncRNA HIF1A-AS1水平明显降低,差异有统计学意义(P<0.05)。见表4。
表4 正常妊娠组、子痫前期组胎盘组织LncRNA HIF1A-AS1、HIF1A mRNA水平比较![]()
Table 4 Comparison of LncRNA HIF1A-AS1 and HIF1A mRNA levels in the placental tissues of normal pregnancy group and preeclampsia group ![]()
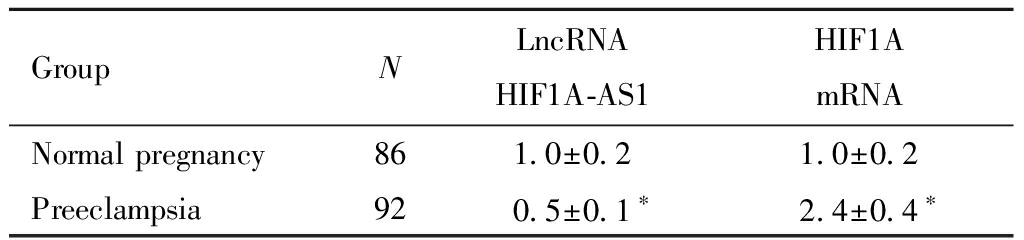
GroupNLncRNA HIF1A-AS1HIF1A mRNANormal pregnancy861.0±0.21.0±0.2Preeclampsia920.5±0.1∗2.4±0.4∗
Note:Compared with the normal pregnancy group,*P<0.05
四、晚发型重度子痫前期孕妇胎盘组织LncRNA HIF1A-AS1、HIF1A mRNA水平与各参数的相关性
Pearson法分析结果显示,晚发型重度子痫前期孕妇胎盘组织LncRNA HIF1A-AS1与HIF1A mRNA水平呈负相关(r=-0.628,P<0.05),且LncRNA HIF1A-AS1与平均管腔面积呈正相关(P<0.05),与平均管壁厚度、收缩压、舒张压均呈负相关(P<0.05);HIF1A mRNA与平均管腔面积呈负相关(P<0.05),与平均管壁厚度、收缩压、舒张压均呈正相关(P<0.05)。见图1、表5。

图1 晚发型重度子痫前期孕妇胎盘组织LncRNA HIF1A-AS1与HIF1A mRNA水平的相关性
Fig. 1 Correlation betweenLNcRNA HIF1A-AS1 and HIF1A mRNA levels in the placental tissue of late onset severe preeclampsia
表5 晚发型重度子痫前期孕妇胎盘组织LncRNA HIF1A-AS1、HIF1A mRNA水平与各参数的相关性
Table 5 Correlation between LncRNA HIF1A-AS1 and HIF1A mRNA levels in the placental tissue of late onset severe preeclampsia pregnant women with different parameters

IndexAverage pipe wall thicknessAverage lumen areaSystolic pressureDiastolic pressureLncRNA HIF1A-AS1r-0.516 0.510-0.524-0.498P 0.006 0.004 0.008 0.012HIF1A mRNAr 0.497-0.489 0.521 0.530P 0.008 0.010 0.006 0.002
讨 论
子痫前期是妊娠期高血压的一种,其主要临床表现为妊娠中期高血压、蛋白尿及水肿,不仅会造成母体全身多器官功能紊乱,而且会对母婴生命健康造成威胁[8]。子痫前期发病机制有母婴免疫失衡、血管内皮损伤、胚盘浅着床、胎盘螺旋动脉重铸不足及滋养细胞侵入不足等[9-10]。本研究将探究筛选出的LncRNA HIF1A-AS1、HIF1A两指标与晚发型重度子痫前期胎盘螺旋动脉重铸的关系。
正常妊娠状态下,螺旋动脉血管中层弹力纤维和肌纤维消失,进而被无收缩功能的纤维蛋白样物质取代,使得管壁变薄、管腔增大[11]。子宫螺旋动脉重铸是母体血管内皮、免疫细胞与胎盘滋养细胞相互作用完成的[12]。而本研究显示晚发型重度子痫前期孕妇平均管壁厚度明显高于正常妊娠孕妇,平均管腔面积明显低于正常妊娠孕妇,进一步提示子宫动脉处于管壁厚、管径小的结构,使得阻力大、血流小,出现“高阻低流”的状态,导致胎盘灌注不足,进而可能影响晚发型重度子痫前期的发生发展。
HIF1A是一种低氧环境诱导的转录因子,可通过低氧反应原件定向结合在靶基因的启动子上,进而调控下游一系列基因的转录[13-14]。研究表明,HIF1A参与肿瘤坏死因子、转化生长因子、血管内皮生长因子及胎盘生长因子等细胞因子的调控,而这些因子均能参与滋养细胞的分化,影响滋养层细胞凋亡及浸润能力[15-16]。Wu等[17]研究显示,LncRNA HIF1A-AS2能间接调节滋养层细胞侵袭和增殖。本研究结果显示,与正常妊娠孕妇相比,子痫前期孕妇胎盘组织HIF1A mRNA水平明显升高,LncRNA HIF1A-AS1水平明显降低,且二者呈明显负相关。提示LncRNA HIF1A-AS1、HIF1A可能在晚发型重度子痫前期的发生发展中起重要作用,基于二者特殊的调控机制,推测病理状态下LncRNA HIF1A-AS1表达降低,LncRNA HIF1A-AS1调控的HIF1A水平升高,HIF1A通过影响其下游细胞因子的表达,进一步参与晚发型重度子痫前期的发生发展。何美荣等[18]研究结果显示,HIF1A在子痫前期患者胎盘中异常高表达,且其作用机制与本研究推测基本一致。本研究进一步采用Pearson法分析LncRNA HIF1A-AS1、HIF1A与各参数的相关性,结果中LncRNA HIF1A-AS1、HIF1A与平均管壁厚度、平均管腔面积、收缩压、舒张压均具有相关性。进一步推测LncRNA HIF1A-AS1、HIF1A调控的下游细胞因子表达增加后,可抑制滋养层细胞分化,使得胎盘滋养层细胞浸润能力下降,对螺旋动脉浸润不足,进而导致螺旋动脉重铸不足,使孕妇出现一系列子痫前期的临床表现,血压异常,收缩压和舒张压升高。
综上所述,晚发型重度子痫前期孕妇胎盘组织中LncRNA HIF1A-AS1低表达,HIF1A mRNA高表达,二者均与螺旋动脉管壁厚度和管腔面积具有相关性。LncRNA HIF1A-AS1、HIF1A可能通过参与螺旋动脉重铸不足,影响晚发型重度子痫前期的发生发展。本研究可为今后晚发型重度子痫前期发病机制的进一步探索提供方向。但LncRNA HIF1A-AS1是否能通过调节HIF1A的表达参与胎盘螺旋动脉重铸不足以诱导子痫前期发生,目前仍不十分明确,其相关调节机制今后将进一步通过动物和细胞试验验证。此外,本研究并未纳入34周前发病的早发型子痫前期患者,未能对比早发型重度子痫前期患者与晚发型重度子痫前期患者胎盘结果是否有差异,今后将增加样本量进一步探究。
1 杨怡珂,漆洪波.美国妇产科医师学会(ACOG)“妊娠期高血压和子痫前期指南2019版”要点解读(第一部分).中国实用妇科与产科杂志,2019,35:895-899.
2 Yang Y,Li H,Ma Y,et al.MiR-221-3p is down-regulated in preeclampsia and affects trophoblast growth,invasion and migration partly via targeting thrombospondin 2.Biomed Pharmacother,2019,109:127-134.
3 张翠翠,谢玲,李洁.重度子痫前期患者血清和胎盘VEGF、sVEGFR-1水平异常与lncRNA-LOC391533表达的相关性分析.临床和实验医学杂志,2019,18:2293-2296.
4 Xue X,Luo L.LncRNA HIF1A-AS1 contributes to ventricular remodeling after myocardial ischemia/reperfusion injury by adsorption of microRNA-204 to regulating SOCS2 expression.Cell Cycle,2019,18:2465-2480.
5 Hong F,Gao Y,Li Y,et al.Inhibition of HIF1A-AS1 promoted starvation-induced hepatocellular carcinoma cell apoptosis by reducing HIF-1α/mTOR-mediated autophagy.World J Surg Oncol,2020,18:113.
6 李山,蒋向阳,刘顺达.低氧诱导因子-1α、血管内皮生长因子、Bcl-2与急性脑梗死患者神经功能缺损度的相关研究.神经损伤与功能重建,2019,14:79-82.
7 谢幸,孔北华,段涛.妇产科学.9版.北京:人民卫生出版,2018:105-109.
8 Sebastian A,Raj T,Yenuberi H,et al.Angiogenic factors and uterine artery Doppler in predicting preeclampsia and associated adverse outcomes in a tertiary hospital in south India.Pregnancy Hypertens,2019,16:26-30.
9 Wang L,Zhang Y,Qu H,et al.Reduced ELABELA expression attenuates trophoblast invasion through the PI3K/AKT/mTOR pathway in early onset preeclampsia.Placenta,2019,87:38-45.
10 Zhang Y,He XY,Qin S,et al.Upregulation of PUM1 expression in preeclampsia impairs trophoblast invasion by negatively regulating the expression of the lncRNA HOTAIR.Mol Ther,2020,28:631-641.
11 Allerkamp HH,Clark AR,Lee TC,et al.Something old,something new:digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation.Hum Reprod,2021,36:571-586.
12 徐欣然,王妍平,崔洪艳.胎盘外泌体在子宫螺旋动脉重铸不全相关妊娠并发症中的作用.国际妇产科学杂志,2019,46:288-292.
13 Kosovic I,Prusac IK,Mestrovic Z,et al.HIF-1α immunohistochemical expression in decidual cells,villous and extravillous trophoblast in placentas from pregnancies complicated with preeclampsia.Pregnancy Hypertens,2020,21:176-178.
14 Vetrovoy OV,Nimiritsky PP,Tyulkova EI,et al.The content and activity of hypoxia-inducible factor HIF1α increased in the hippocampus of newborn rats that were subjected to prenatal hypoxia on days 14-16 of embryogenesis.Neurochem J,2020,14:286-289.
15 徐峰,王莹,鲁晓燕,等.HIF-1α、TLR4与稽留流产妊娠滋养细胞凋亡的关系研究.中国计划生育和妇产科,2020,12:73-77.
16 Albers RE,Kaufman MR,Natale BV,et al.Trophoblast-Specific Expression of Hif-1α Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction.Sci Rep,2019,9:2742.
17 Wu D,Yang N,Xu Y,et al.lncRNA HIF1A antisense RNA 2 modulates trophoblast cell invasion and proliferation through upregulating PHLDA1 expression.Mol Ther Nucleic Acids,2019,16:605-615.
18 何美荣,王彩珊.缺氧诱导因子HIF-1a在子痫前期患者胎盘中的表达.中国保健营养,2019,29:292.