卵母细胞是决定辅助生殖技术(assisted reproductive technology,ART)成功与否的最重要因素之一。卵母细胞的质量不但与受精率密切相关,而且能够明显地影响后续形成的胚胎的发育能力[1]。客观准确地评估卵母细胞质量,帮助胚胎学家选择出具有优越发育能力的胚胎,才能最终提高辅助生殖的临床效率。本研究的目的是通过评估ICSI周期大极体卵母细胞的受精、胚胎发育及妊娠结局,观察卵母细胞大极体是否是影响胚胎质量的预测因素,从而辅助移植胚胎选择,以帮助其改善ART后的妊娠结局。
对象与方法
一、研究对象
本研究回顾性分析华中科技大学同济医学院附属同济医院生殖医学中心2017年1月—2021年12月行辅助生殖助孕的不孕症患者的临床资料,将ICSI中含有大极体卵母细胞的112个新鲜周期作为研究对象,并将ICSI注射的880枚成熟卵母细胞(MII)根据第一极体大小分为大极体组和正常极体组,见图1,分别比较两组胚胎的受精、发育潜能和临床结局。纳入标准:(1)ICSI周期;(2)女方年龄≤40岁;(3)至少含有1枚大极体卵母细胞。本研究通过本院医学伦理委员会批准,所有患者夫妻双方均了解并知情同意。

图1 第一极体的形态(×200,比例尺=20 μm)
Figure 1 Morphology of the first polar body(×200, scale bar=20 μm)
二、研究方法
1.卵母细胞收集及脱颗粒:按本中心常规方案促排卵,肌肉注射人绒毛膜促性腺激素(human chorionic gonadotropin,HCG)10 000 U,36 h后在阴道超声监测下穿刺取卵。取出卵母细胞后,将卵冠丘复合物(cumulus oocyte complexes,COCs)置培养箱中培养(37 ℃,6%CO2),获卵后2~4 h按采卵的先后顺序用80 IU/mL的透明质酸酶(vitrolife,瑞典)消化COCs,并用stripper(140 um,cook,美国)机械法去除颗粒细胞,去除颗粒细胞后置于G-1卵裂培养液(Vitrolife,瑞典)中培养。
2.ICSI注射及第一极体形态评估:在HCG给药后36~41 h进行ICSI注射,ICSI注射时在显微镜下对MII卵母细胞第一极体大小进行评估并详细记录,146枚第一极体明显增大的MII卵母细胞为大极体组,734枚第一极体大小正常的MII卵母细胞为正常极体组。ICSI受精后将胚胎置于G-1卵裂培养液(Vitrolife,瑞典)中,在37 ℃,6.0% CO2,5.0% O2,89.0% N2的EmbryoScopeTM延时监测(time-lapse monitoring,TLM)培养箱(Vitrolife,瑞典)中进行体外胚胎培养。
3.胚胎评估:16~18 h评估卵母细胞受精情况并记录原核(pronucleus,PN)数目:2PN为正常受精;NPN为未受精;1PN和≥3PN分别为 1个原核和≥3个原核的异常受精。
本中心D2/D3卵裂胚评分标准:1级,卵裂球大小均匀且碎片≤5%;2级,卵裂球大小均匀或稍不均匀≤20%;3级,卵裂球大小严重不均且碎片<50%,或卵裂球无大小严重不均、碎片20%~50%;4级,胚胎卵裂球少,碎片≥50%。2PN卵裂指的是D1为正常受精(2PN),D2卵裂球数为≥2个。D3优质胚胎标准:D1为正常受精(2PN),D2卵裂球数为 4~5个,D3卵裂球数7~10个,卵裂球大小均匀且碎片<10%。本中心常规将D3 移植胚胎以外剩余的胚胎继续培养,并采用 Gardner 囊胚评分系统[2]根据囊胚腔扩张程度、内细胞团(inner cell mass,ICM)和滋养层细胞(trophectoderm,TE)的形态和数目对囊胚进行质量评估。冷冻囊胚标准:D5/D6囊胚评分≥3/4 BC。优质囊胚标准:D5/D6囊胚评分≥3/4 BB。
4.妊娠结局的评估:胚胎移植后12~14 d进行HCG检测。临床妊娠为胚胎移植后至少5周行阴道B超可见孕囊,包括宫外孕;活产是指胎儿娩出后至少具有生命体征心跳、呼吸、脐带搏动、随意肌收缩之一;临床妊娠后20周内无胎心活动为流产;生化妊娠指胚胎移植4~6周HCG阳性,但阴道B超未见到孕囊。
5.统计学分析:采用SPSS 26.0 统计学软件包进行统计分析,符合正态分布的计量资料数据用均数±标准差![]() 表示,组间均数比较采用t检验,率与构成比的组间比较采用卡方检验,多因素Logistic回归分析各因素对受精和胚胎发育的影响。以P<0.05为差异具有统计学意义。
表示,组间均数比较采用t检验,率与构成比的组间比较采用卡方检验,多因素Logistic回归分析各因素对受精和胚胎发育的影响。以P<0.05为差异具有统计学意义。
结 果
一、两组基础资料的比较:大极体组卵泡刺激素(follicle-stimulating hormone,FSH)高于正常极体组(P<0.05),两组的女方年龄、体质指数(body mass index,BMI)、不孕类型、不孕年限比较均无统计学差异(P>0.05)。见表1。
表1 两组基本情况比较
Table 1 Comparison of the two groups of basic information
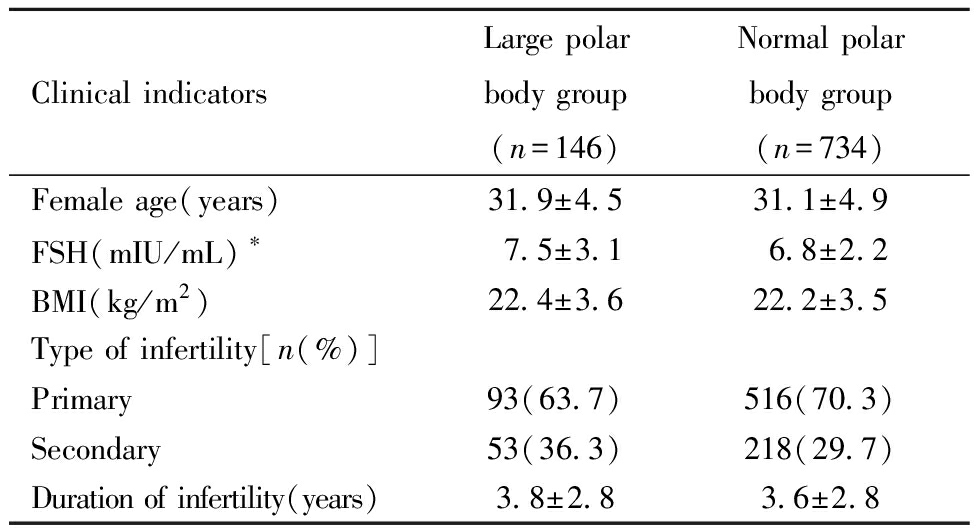
Clinical indicatorsLarge polar body group(n=146)Normal polar body group(n=734)Female age(years)31.9±4.531.1±4.9FSH(mIU/mL)∗7.5±3.16.8±2.2BMI(kg/m2)22.4±3.622.2±3.5Type of infertility[n(%)]Primary93(63.7)516(70.3)Secondary53(36.3)218(29.7)Duration of infertility(years)3.8±2.83.6±2.8
*P<0.05,FSH:follicle-stimulating Hormone;BMI:body mass index.
二、两组受精和胚胎发育情况比较:大极体组2PN受精率显著低于正常极体组(P<0.05);≥3PN率显著高于正常极体组(P<0.01);1PN率和退化率(degradation rate,DA)呈升高趋势,但均无统计学差异(P>0.05)。与正常极体组相比,大极体组2PN卵裂率呈下降趋势,D3优胚率、囊胚形成率、可利用囊胚率、优质囊胚率均明显降低,但仅囊胚形成率有统计学差异(P<0.01)。见表2。
表2 两组受精和胚胎发育情况比较
Table 2 Comparison of the fertilization and embryo development between the two groups[n(%)]
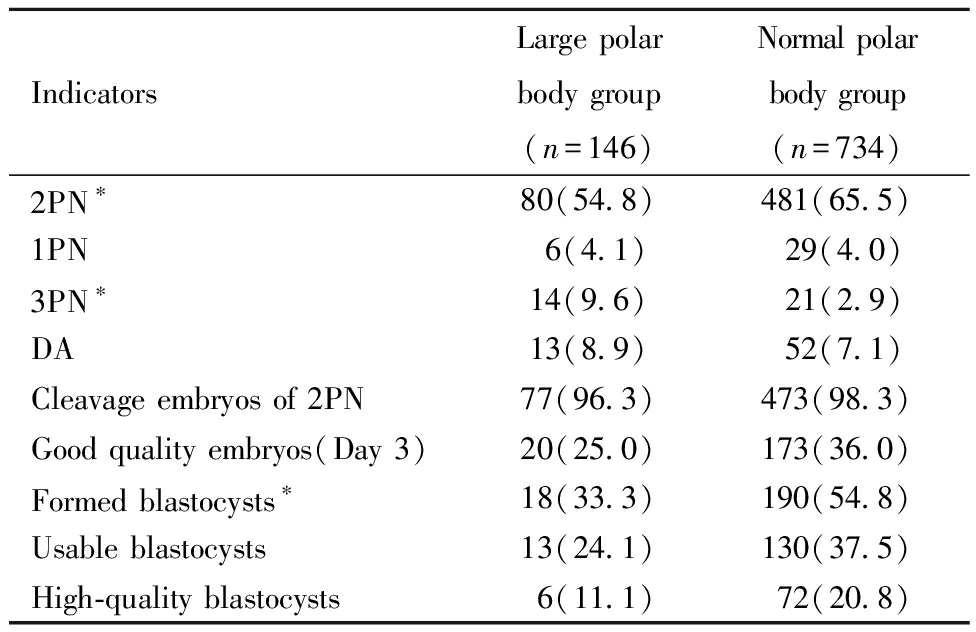
IndicatorsLarge polar body group(n=146)Normal polar body group(n=734)2PN∗80(54.8)481(65.5)1PN6(4.1)29(4.0)3PN∗14(9.6)21(2.9)DA13(8.9)52(7.1)Cleavage embryos of 2PN77(96.3)473(98.3)Good quality embryos(Day 3)20(25.0)173(36.0)Formed blastocysts∗18(33.3)190(54.8)Usable blastocysts13(24.1)130(37.5)High-quality blastocysts6(11.1)72(20.8)
*P<0.05, #P<0.01; PN:pronucleus; DA:degradation.
三、多因素Logistic回归分析各因素对受精和胚胎发育的影响:Logistic回归结果显示,考虑了女方年龄、FSH、BMI、不孕类型、不孕年限等混杂因素后,与正常极体组相比,大极体组2PN受精率显著降低(OR:0.63,95%CI:0.44~0.91,P<0.05);≥3PN率显著增高(OR:3.99,95%CI:1.95~8.16,P<0.001);D3优胚率(OR:0.54,95%CI:0.31~0.93,P<0.05)和囊胚形成率(OR:0.42,95%CI:0.23~0.78,P<0.01)显著降低。见表3。
表3 多因素Logistic回归分析卵母细胞大极体对受精和胚胎发育的影响
Table 3 Effects of oocytes with large polar bodies on fertilization and embryo development by logistic regression analysis
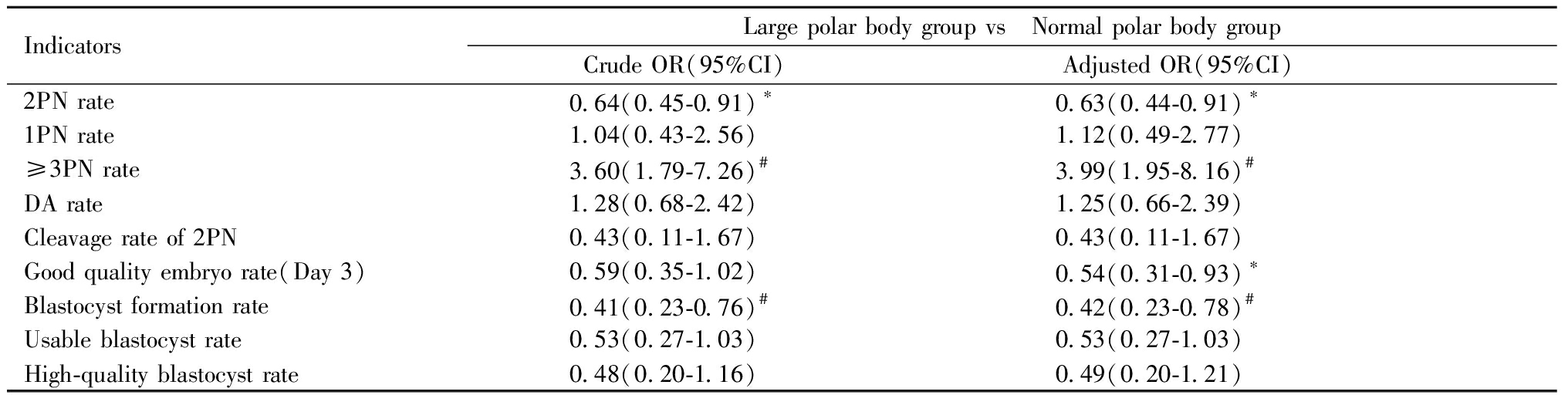
IndicatorsLarge polar body group vs Normal polar body groupCrude OR(95%CI) Adjusted OR(95%CI) 2PN rate0.64(0.45-0.91)∗0.63(0.44-0.91)∗1PN rate1.04(0.43-2.56)1.12(0.49-2.77)≥3PN rate 3.60(1.79-7.26)#3.99(1.95-8.16)#DA rate1.28(0.68-2.42)1.25(0.66-2.39)Cleavage rate of 2PN0.43(0.11-1.67)0.43(0.11-1.67)Good quality embryo rate(Day 3)0.59(0.35-1.02)0.54(0.31-0.93)∗Blastocyst formation rate0.41(0.23-0.76)#0.42(0.23-0.78)#Usable blastocyst rate0.53(0.27-1.03)0.53(0.27-1.03)High-quality blastocyst rate0.48(0.20-1.16)0.49(0.20-1.21)
Compared with the normal polar body group,*P<0.05,#P<0.01. PN:pronucleus; DA:degradation。
四、妊娠结局随访:以新鲜周期移植D3卵裂期胚胎进行比较,大极体组移植一个大极体来源的胚胎4例,临床妊娠1例,流产1例;分别来源于大极体组和正常极体组的两个胚胎联合移植8例,均未妊娠。正常极体组单个胚胎移植23例,临床妊娠12例,活产8例,流产4例,来源于正常极体组的两个胚胎联合移植6例,临床妊娠3例,活产2例,流产1例。复苏移植大极体来源的囊胚共6例,3例移植一个大极体来源囊胚,3例联合移植来源于大极体组和正常极体组的两个胚胎,2例(1例大极体组,1例大极体组+正常极体组)临床妊娠并活产2个健康婴儿。见表4。
表4 临床结局比较
Table 4 Comparison of the clinical outcomes[n(%)]

GroupSingle embryo transferLarge polarbody groupNormal polarbody groupCombined embryo transferLarge polar body group and normal polar body groupNormal polar body group and normal polar body groupNumber of cycles42386Clinical pregnancy1(25.0)12(52.2)03(50.0)Live birth08(34.8)02(33.3)Miscarriage1(25.0)4(17.4)01(16.7)Biochemical pregnancy0000
讨 论
初级卵母细胞第一次减数分裂后形成次级卵母细胞和第一极体,第一极体的排出代表着卵母细胞细胞核的成熟。ICSI之前去除卵丘细胞可以更好地观察第一极体是否存在及其形态,而促性腺激素刺激卵巢后获得的卵母细胞可能经历了不同的细胞质和核成熟时间,因此,第一极体的形态差异可能反映了卵母细胞的核成熟度及其与胞浆发育的同步性[3]。目前,第一极体形态是否可以预测卵母细胞的质量及对辅助生殖技术成功率的影响尚存在争议,有研究表明,选择第一极体形态良好的胚胎移植,受精率更高,胚胎质量更好,着床率和妊娠率更高[4-5],而另一些研究则报道了相互矛盾的结果[6-8]。本研究的目的是通过评估第一极体明显增大的卵母细胞和受精、胚胎发育及妊娠结局之间的关系,观察卵母细胞大极体是否是影响胚胎质量的预测因素,从而辅助移植胚胎选择,以改善ICSI周期ART后的妊娠结局。
卵母细胞中普遍存在大极体现象,卵母细胞大极体发生率为0.5%~2%。Choi等[9]最早在小鼠中发现Mos/丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)途径可以调节第一极体的大小。卵母细胞胞质的不对称分裂依赖于皮层纺锤体定位、皮层重组和卵母细胞极化后的纺锤体迁移[10]。敲除mos基因的小鼠生育力下降,成熟的MOS-/-卵母细胞在第一次减数分裂过程中不能激活MAPK,纺锤体形状发生改变,纺锤体定位失败不能迁移到皮层,导致卵裂面改变,产生异常大的第一极体。Huang等[11]研究发现肌动蛋白、细胞膜、微管相关WASP同源体(WASP homolog associated with actin, membranes and microtubules,WHAMM)是小鼠卵母细胞成熟外周纺锤体迁移和不对称细胞质分裂所必需的。卵母细胞细胞质中微量注射特异性短干扰(si)RNA后WHAMM被耗尽,肌动蛋白帽的形成被中断,纺锤体变大并停留在中部,纺锤体迁移失败导致卵母细胞减数分裂出现对称细胞质分裂,卵母细胞挤压出较大的第一极体。
很多研究报道了人卵母细胞大极体与受精率、卵裂率、胚胎发育之间的关系。Ebner等[5]报道了66例患者70个ICSI周期的610枚MII卵母细胞第一极体形态对受精率和胚胎质量的影响,结果表明,大极体卵母细胞(18例)与其它组卵母细胞(完整平滑组,完整粗糙组,破碎组)相比,2PN受精率明显下降(50.0%),D2胚胎质量(胚胎碎片<25%)明显下降(22.2%),建议第一极体增大的卵母细胞不应该考虑移植。Fancsovits等[12] 回顾性研究了522例患者的3387枚MII卵母细胞的第一极体形态与ICSI周期受精和胚胎发育的相关性,与其它组(完整平滑组,完整粗糙组,破碎组,未成熟组)相比,大极体卵母细胞组(62例)2PN受精率最低(50.0%),且大极体组D2多核胚胎数(26.7%)和胚胎碎片(20.5±12.1)显著高于其它组,不应该选择大极体卵母细胞发育的胚胎进行移植。Navarro等[13]回顾性分析了3177枚MII卵母细胞第一极体形态对ICSI结局的影响,大极体卵母细胞组(363例)的2PN受精率、D2卵裂率和胚胎质量(分别为20.7%、18.7%和5.0%)均明显低于正常大小完整的极体组和极体破碎组,表明具有大极体的卵母细胞不应用于ICSI手术。李澎涛等[14]分析了63个ICSI周期的765枚MII卵母细胞第一极体的形态与卵母细胞的受精率、卵裂和发育潜能的关系,与极体完整组和极体破碎组相比,大极体卵母细胞组(49例)的正常受精率、卵裂率、优质胚胎率明显下降(分别为67.4%、84.9%和3.3%),异常受精率明显增高(14.3%),第一极体形态可以作为评价人类卵母细胞发育潜能的指标之一。
在本研究中,我们回顾性分析了ICSI周期卵母细胞大极体与受精率、胚胎发育的关系,与正常极体组相比,大极体组2PN正常受精率显著降低;D3优胚率、囊胚形成率、可利用囊胚率、优质囊胚率均明显降低,但仅囊胚形成率有统计学差异。考虑混杂因素后,大极体卵母细胞2PN正常受精率显著降低(OR=0.63);D3优胚率(OR=0.54)和囊胚形成率(OR=0.42)均显著减低。卵母细胞大极体是影响正常受精、D3胚胎质量和囊胚形成的危险因素,这与之前其它研究报道卵母细胞大极体影响受精及胚胎后期发育一致[5,12-14]。卵母细胞第一次减数分裂时纺锤体的异常导致同源染色体分离不均,第一极体中可能含有非整倍染色体,次级卵母细胞非整倍率增加[15]。在陈雯、贾婵维等[16-17]的研究中大极体的卵母细胞非整倍体发生率明显增高,与本研究大极体卵母细胞异常受精风险(≥3PN率调整后OR=3.99)明显增高相一致。同时纺锤体定位迁移失败使纺锤体位于卵母细胞中央,卵裂面扩大导致胞浆不对称分裂被破坏,第一极体划走一大部分胞浆形成大极体,次级卵母细胞中细胞器、细胞因子和蛋白质大量减少,最终影响卵母细胞受精及胚胎后期发育。鉴于以往研究未分析临床结局,本研究进一步随访妊娠结局,新鲜周期移植大极体来源卵裂期胚胎共12例(大极体组4例,大极体组+正常极体组8例),1例临床妊娠后流产,余均未妊娠;复苏移植大极体来源的囊胚共6例(大极体组3例,大极体组+正常极体组3例),2例(大极体组1例,大极体组+正常极体组1例)临床妊娠并活产2个健康婴儿。因此,不建议移植大极体卵母细胞来源的卵裂期胚胎,若只有大极体来源的胚胎,建议将胚胎培养至囊胚后再移植,可能会改善其临床结局。
综上所述,在ICSI周期中,卵母细胞大极体会影响受精及胚胎后期发育,可以将卵母细胞出现大极体作为预测胚胎质量的因素,并将其作为选择移植胚胎的一个参考指标,这样有助于进一步改善ICSI周期移植的妊娠结局。
1 Lasiene K,Vitkus A,Valanciūte A,et al.Morphological criteria of oocyte quality.Medicina(Kaunas),2009,45:509-515.
2 Gardner DK,Lane M,Stevens J,et al.Blastocyst score affects implantation and pregnancy outcome:towards a single blastocyst transfer.Fertil Steril,2000,73:1155-1158.
3 Eichenlaub-Ritter U,Schmiady H,Kentenich H,et al.Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient:indicators of asynchrony in nuclear and cytoplasmic maturation.Hum Reprod,1995,10:2343-2349.
4 Ebner T,Moser M,Yaman C,et al.Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy.Fertil Steril,1999,72:599-603.
5 Ebner T,Yaman C,Moser M,et al.Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection.Hum Reprod,2000,15:427-430.
6 Verlinsky Y,Lerner S,Illkevitch N,et al.Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? Reprod Biomed Online,2003,7:336-341.
7 De Sutter P,Dozortsev D,Qian C,et al.Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection.Hum Reprod,1996,11:595-597.
8 Balaban B,Urman B,Sertac A,et al.Oocyte morphology does not affect fertilization rate,embryo quality and implantation rate after intracytoplasmic sperm injection.Hum Reprod,1998,13:3431-3433.
9 Choi T,Fukasawa K,Zhou R,et al.The Mos/mitogen-activated protein kinase(MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes.Proc Natl Acad Sci U S A,1996,93:7032-7035.
10 Coticchio G,CantoMD,Renzini MM,et al.Oocyte maturation:gamete-somatic cells interactions,meiotic resumption,cytoskeletal dynamics and cytoplasmic reorganization.Hum Reprod Update,2015,21:427-454.
11 Huang X,Ding L,Pan R,et al.WHAMM is required for meiotic spindle migration and asymmetric cytokinesis in mouse oocytes.Histochem Cell Biol,2013,139:525-534.
12 Fancsovits P,Tothne ZG,Murber A,et al.Correlation between first polar body morphology and further embryo development.Acta Biol Hung,2006,57:331-338.
13 Navarro PA,de Araújo MM,de Araújo CM,et al.Relationship between first polar body morphology before intracytoplasmic sperm injection and fertilization rate,cleavage rate,and embryo quality.Int J Gynaecol Obstet ,2009,104:226-229.
14 李澎涛,王娜,殷晨星,等.卵母细胞第一极体形态对受精结局的影响.广东医学,2016,37:3732-3734.
15 Sun SC,Kim NH.Molecular Mechanisms of Asymmetric Division in Oocytes.Microsc Microanal,2013,19:883-897.
16 陈雯,Schmutzler A,Weimer J,等.第一极体形态学与人类卵细胞非整倍体相关关系的研究.现代妇产科进展杂志,2003,12:321-323.
17 贾婵维,梁毓,王树玉.IVF-ET周期中第一极体形态与未受精卵母细胞非整倍体相关性研究.中国优生与遗传杂志,2010,18:125.